2025.10.14 Tuesday
Calcium Could Be Key to Solving Stability Issues in Sodium-Ion Batteries
Researchers demonstrate that calcium doping prevents the degradation of the cathode in air and water
Rechargeable batteries are a fundamental part of today's technological landscape, powering everything from our personal devices to large-scale infrastructure. While many types of rechargeable batteries exist, lithium-ion batteries (LIBs) are by far the most ubiquitous, owing to their outstanding energy density, long life cycle, and low self-discharge rate. However, lithium is rather a scarce element with a very uneven distribution throughout the world, prompting research into batteries made from other materials.
Over the past decade, scientists have focused strongly on sodium-ion batteries (SIBs), which offer a compelling alternative to LIBs. The main advantage of SIBs is the abundance of sodium, which is present in seawater and is much more affordable and safer than lithium. This makes SIBs a promising option for large-scale energy storage, such as for renewable energy grids. Despite these promising qualities, SIBs have their drawbacks. A major challenge is the stability of the cathode material in air and water, which can degrade the battery’s performance and lifespan.
In a recent study, a research team led by Professor Shinichi Komaba, Assistant Professors Zachary T. Gossage and Changhee Lee, Project Scientist Shinichi Kumakura from Tokyo University of Science (TUS), Japan, has made substantial progress toward addressing this pressing limitation of SIBs. Their study was co-authored by Ms. Monalisha Mahapatra, a second-year doctoral student from TUS, who has made remarkable contributions to this research. Their paper, published online in the Journal of Materials Chemistry A on August 29, 2025, reports a new way of enhancing the air and water stability of Na2/3[Fe1/2Mn1/2]O2 (NFM), a very promising composition for the cathode material of P2-type SIBs.
Their approach centers around replacing some of the sodium (Na) ions in NFM with calcium (Ca) ions--a technique known as ‘doping.’ Even though the final concentration of Ca ions is low compared to the overall weight of the electrode (less than 2%), doping can have a marked impact on various properties. Experiments showed that Ca-doped NFM had a higher rate of performance than regular NFM while maintaining a high discharge capacity. Most importantly, Ca-doped NFM exhibited high stability in air and water. Whereas 2 days of air exposure made regular NFM lose 35% of its discharge capacity, no losses were observed in Ca-doped NFM.
To understand the underlying reasons behind these improvements, the team conducted a detailed analysis, revealing the interesting behavior of Ca in NFM. “According to our surface analyses, the improved stability appears to stem from spontaneous Ca migration during air exposure, which leads to the development of a protective Ca-enriched surface layer that suppresses the decomposition processes, such as Na+/H+ exchange and Na deintercalation,” explains Prof. Komaba. “This newly explored mechanism appears to be quite effective for mitigating surface degradation reactions in layered oxides.” The team also noted that Ca doping improved crystallinity and increased interlayer spacing in NFM, which contributes to a better electrochemical performance. Also, the protective layer shields the NFM during storage before battery assembly.
By discovering the protective effects of Ca doping in NFM, this study could pave the way to the widespread adoption of SIBs. Ca materials are abundantly available and they can be easily incorporated into NFM using synthetic techniques, without increasing the cost substantially. This would significantly benefit renewable energy generation by providing a more sustainable and cost-effective solution for large-scale energy storage. Furthermore, it would solve lithium scarcity issues, ensuring a stable supply chain for rechargeable batteries for a wide range of electric and electronic devices. “I believe that the outstanding results achieved in such a short period of time by Ms. Monalisha Mahapatra, a student from India through JICA, were due to both her own hard work and the support system of the entire laboratory,” highlights Prof. Komaba.
Future studies in this field will hopefully allow researchers to tap into the full potential of doping in SIBs.
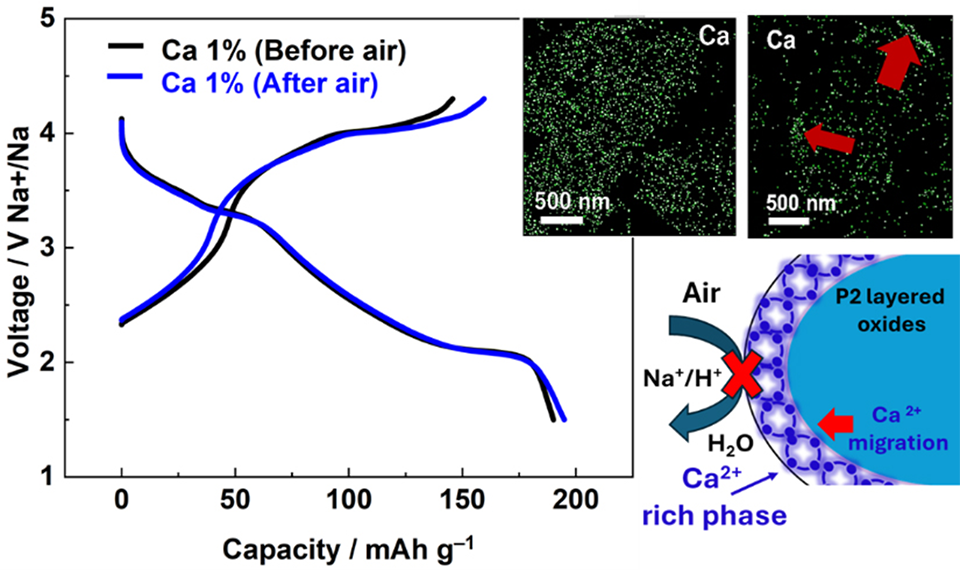
Image title: Calcium-doped NFM to resolve stability issues in sodium-ion batteries
Image caption: By introducing a minuscule amount of calcium into the sodium-ion layer of NFM, a promising cathode material, its stability greatly improved.
Image credit: Professor Shinichi Komaba from Tokyo University of Science, Japan
Image link: https://doi.org/10.1039/D5TA04742K
License type: CC BY-NC 3.0
Usage restrictions: Credit must be given to the creator. Only non-commercial uses of the work are permitted.
Reference
| Title of original paper | : | Enhanced air stability by calcium doping in Na2/3[Fe1/2Mn1/2]O2 cathode material for Na-ion batteries |
| Journal | : | Journal of Materials Chemistry A |
| DOI | : | 10.1039/D5TA04742K |
About The Tokyo University of Science
Tokyo University of Science (TUS) is a well-known and respected university, and the largest science-specialized private research university in Japan, with four campuses in central Tokyo and its suburbs and in Hokkaido. Established in 1881, the university has continually contributed to Japan's development in science through inculcating the love for science in researchers, technicians, and educators.
With a mission of "Creating science and technology for the harmonious development of nature, human beings, and society," TUS has undertaken a wide range of research from basic to applied science. TUS has embraced a multidisciplinary approach to research and undertaken intensive study in some of today's most vital fields. TUS is a meritocracy where the best in science is recognized and nurtured. It is the only private university in Japan that has produced a Nobel Prize winner and the only private university in Asia to produce Nobel Prize winners within the natural sciences field.
■
Tokyo University of Science(About TUS)

About Professor Shinichi Komaba
from Tokyo University of Science
Dr. Shinichi Komaba is currently a Professor in the Department of Applied Chemistry at Tokyo University of Science (TUS). He obtained his Ph.D. from Waseda University in Japan. At TUS, he also leads the Komaba lab, which focuses on the development of next-generation energy-storage materials. He has published over 490 articles, which have received over 40,000 citations. His research primarily centers around sodium-ion batteries, with a broader focus on functional solid-state chemistry, inorganic industrial materials, and electrochemistry. He has received multiple awards for his contributions, including the "Wiley Top viewed article" in 2023.
About Assistant Professor Gossage Zachary Tyson
from Tokyo University of Science
About Assistant Professor Lee Changhee
from Tokyo University of Science
Funding information
This study was partially funded by the Ministry of Education, Culture, Sports, Science and Technology (MEXT) Program: Data Creation and Utilization Type Materials Research. (JPMXP1122712807), the JST through CREST (Grant No. JPMJCR21O6), ASPIRE (JPMJAP2313), GteX (JPMJGX23S4), and JSPS KAKENHI Grant Numbers JP25H00905, JP24H00042, and JP20H02849.

