2026.01.27 Tuesday
A New Route to Synthesize Multiple Functionalized Carbon Nanohoops
Researchers develop a versatile molecular platform that enables multi-site functionalization and creation of chiral nanohoops exhibiting advanced photophysical properties
The field of nanomaterials is witnessing a transformative shift at the intersection of organic chemistry and molecular engineering. Among the most promising molecular structures are carbon nanohoops, of which [n]cycloparaphenylenes ([n]CPPs) are a representative example. These ring-shaped structures represent the smallest possible slices of carbon nanotubes, which themselves are a widely renowned material of the 21st century.
Given that their structures can, in principle, be precisely tuned at the atomic level, nanohoops hold great potential as molecular components for next-generation optoelectronic devices, including high-resolution displays, photonic circuits, and responsive sensing materials. This potential has motivated researchers worldwide to develop synthetic strategies that provide precise control over nanohoop construction and functionalization.
Yet, despite their promise, creating nanohoops with multiple functional handles and transforming them into π-extended or chiral derivatives remains a central challenge. Because these molecules have strong ring strain, the construction of the CPP framework itself is far from straightforward, and as a result, introducing substituents at specific positions has been extremely difficult using conventional synthetic methods. Therefore, the development of versatile "platform molecules" that can be diversified into a range of functional nanohoops has been a long-standing goal in the field.
Addressing this challenge, a research team led by Assistant Professor Yoshitaka Tsuchido from the Department of Chemistry, Tokyo University of Science (TUS), Japan, has developed a breakthrough method for creating highly functionalized [n]CPPs. Their study, published online in the journal Angewandte Chemie International Edition on December 30, 2025, describes the creation of a [9]CPP framework--a hoop consisting of nine benzene rings--featuring six strategically placed bromine substituents, and demonstrates how this platform can be diversified into π-extended and chiral nanohoops with remarkable photophysical properties, including high circularly polarized luminescence (CPL) performance. The paper was co-authored by Mr. Naoya Kinoshita, who completed his Master's course in 2022; Ms. Nanami Kotani, a second-year Master's student; Mr. Masaya Sugiura, a first-year Master's student; Mr. Kotaro Matsumura, a third-year Ph.D. student; and Professor Hidetoshi Kawai, all from TUS, together with Professor Daisuke Sakamaki (Osaka Metropolitan University) and Professors Masashi Hasegawa and Daisuke Tauchi (Kitasato University).
A key to this achievement is the gold-mediated macrocyclization strategy developed by one of the authors, which enables the assembly of the strained nanohoop under mild conditions while preserving multiple reactive bromine sites. The target compound was synthesized in five steps with a 37% overall yield from commercially available 1,4-dibromobenzene. Notably, the brominated nanohoop exhibits phosphorescence at low temperatures, attributed to the heavy-atom effect of the bromine substituents, further highlighting the unique characteristics of this molecular scaffold.
Once this bromine-containing platform was established, the researchers showcased its versatility through palladium-catalyzed cross-couplings, which allowed substituents to be introduced at six distinct positions around the nanohoop. This modularity enabled the construction of π-extended nanohoops through six-fold annulation, yielding a chiral nanohoop. This molecule features an armchair-type architecture composed of three dibenz[a,h]anthracene units, giving rise to four atropisomers, which were separated by chiral HPLC. One pair of enantiomers displayed CPL with |glum| = 0.100. This exceptionally large asymmetry factor places it among the highest reported for chiral π-conjugated molecules, demonstrating that late-stage π-extension can produce nano-carbon structures with such outstanding optical performance. "Our results demonstrate that the bromo-functionalized CPP synthesized in this study serves not only as a reactive platform for multi-point derivatization, but also as a gateway to new nanohoops with advanced optical functions," remarks Dr. Tsuchido.
By establishing a reliable and adaptable route to multi-functional nanohoop platforms, and by demonstrating their transformation into CPL-active and π-extended chiral architectures, this research significantly expands the design space of nanohoop-based materials. These could lead to the development of molecular electronic devices and photonic devices, such as energy-efficient 3D displays, more secure optical communication lasers, and even new types of advanced catalysts for chemical manufacturing. Overall, this research marks a definitive step forward in our ability to design and build the precise carbon structures that will power the technology of the near future.
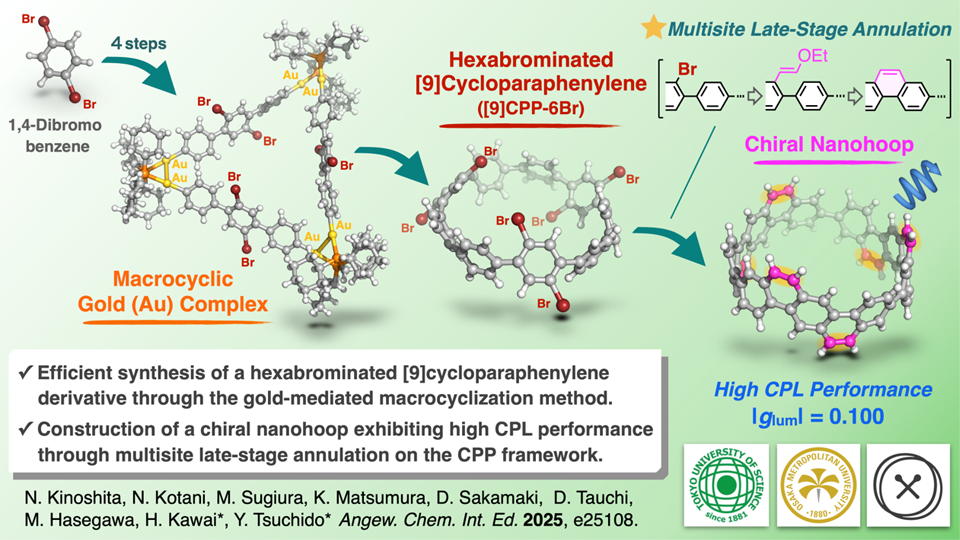
Image title: Synthesis of highly functionalized carbon nanohoops
Image caption: Through the reliable and scalable synthesis procedure reported in this study, it is possible to obtain carbon nanohoops with exceptional functional properties.
Image credit: Dr. Yoshitaka Tsuchido from Tokyo University of Science, Japan
License type: Original content
Usage restrictions: Cannot be used without permission
Reference
| Title of original paper | : | A Precisely Bromo-Functionalized [9]Cycloparaphenylene as a Platform for Late-Stage Multisite π-Extension Toward Chiral Nanohoops |
| Journal | : | Angewandte Chemie International Edition |
| DOI | : | 10.1002/anie.202525108 |
About The Tokyo University of Science
Tokyo University of Science (TUS) is a well-known and respected university, and the largest science-specialized private research university in Japan, with four campuses in central Tokyo and its suburbs and in Hokkaido. Established in 1881, the university has continually contributed to Japan's development in science through inculcating the love for science in researchers, technicians, and educators.
With a mission of "Creating science and technology for the harmonious development of nature, human beings, and society," TUS has undertaken a wide range of research from basic to applied science. TUS has embraced a multidisciplinary approach to research and undertaken intensive study in some of today's most vital fields. TUS is a meritocracy where the best in science is recognized and nurtured. It is the only private university in Japan that has produced a Nobel Prize winner and the only private university in Asia to produce Nobel Prize winners within the natural sciences field.
■
Tokyo University of Science(About TUS)

About Professor Kawai Hidetoshi
from Tokyo University of Science
About Assistant Professor Yoshitaka Tsuchido
from Tokyo University of Science
Dr. Yoshitaka Tsuchido obtained his Engineering and Ph.D degrees from Tokyo Institute of Technology in 2014. He currently serves as an Assistant Professor at Tokyo University of Science, Japan, where he conducts research on π-conjugated macrocyclic compounds, molecular machines, and other areas of organic chemistry. He has published over 40 papers on these topics. In 2025, he received the Incentive Award in Synthetic Organic Chemistry.
Funding information
This work was financially supported by JSPS KAKENHI grant numbers 21K05093 (Grants-in-Aid for Scientific Research (C)), 20H05866, 21H05496, and 23H04041 (Grants-in-Aid for Transformative Research Area (A), Condensed Conjugation). Yoshitaka Tsuchido also acknowledges financial support from Tokuyama Science Foundation and Tokyo University of Science Grant for Young or Female Researchers. Support from the Cooperative Research Program of the "Network Joint Research Center for Materials and Devices," MEXT, Japan, is gratefully recognized.

