2025.06.26 Thursday
Unraveling Protein-Nanoparticle Interactions Using Biophysics
Researchers investigate the interaction dynamics of bovine serum albumin and silica nanoparticles using spectroscopy methods

Nanoparticles (NPs) are materials whose dimensions range from 1 to 1,000 nanometers (nm). Due to their nano-scale dimensions and tunable material properties, NPs have gained interest in the global scientific community in recent years. Applications of NPs in the field of human health include NP-based drug delivery systems and radioactive probe-linked NPs for medical diagnosis. While significant advancements have been achieved in the design and synthesis of NPs, studies investigating the interactions of NPs with important biological macromolecules like proteins remain limited.
To reveal the science behind the protein–nanoparticle interaction and its implications for human health, a team of researchers led by Associate Professor Masakazu Umezawa from the Department of Medical and Robotic Engineering Design, Faculty of Advanced Engineering, Tokyo University of Science, Japan, conducted a series of spectroscopy-based experiments. The research team comprised Mr. Naoya Sakaguchi, a second-year PhD student from the Department of Materials Science and Technology, Faculty of Advanced Engineering, Tokyo University of Science, and Junior Associate Professor Atsuto Onoda from Sanyo-Onoda City University. Their research findings were published online in Langmuir on June 3, 2025.
In their study, the researchers employed bovine serum albumin (BSA) as the main protein of interest and silica NPs (SiNPs) with diameters ranging from 10 nm to 10 μm (10,000 nm). They analyzed the protein–nanoparticle interactions using thioflavin T (ThT) fluorescence, Fourier transform infrared spectroscopy (FT-IR), and circular dichroism (CD).
Explaining the motivation behind the present study, Dr. Umezawa says, "When NPs are administered in vivo, interactions with proteins and other biomolecules may occur, leading to the modulation of their biological effects. Therefore, establishing the safety of NPs along with clarifying the effects of NPs on the secondary structure of proteins is highly important."
The scientists found that the ThT fluorescence intensity decreased with increasing SiNP size. Notably, a drastic increase in the ThT fluorescence intensity was observed when BSA was mixed with 10 nm-sized SiNPs at a stirring time of one hour. However, when BSA was mixed with the largest SiNPs (10 μm) for longer stirring times up to 48 hours, the ThT fluorescence intensity was markedly higher.
"The increase in β-sheet formation in BSA, the most abundant protein in serum and cerebrospinal fluid, is remarkably high during interaction with 10 nm-sized SiNPs. This shows that ultra-small SiNPs can induce abnormal protein conformation and have the potential to cause pathological conditions like Alzheimer's disease, which involves the formation of amyloid β-peptides," states Dr. Umezawa.
Further FT-IR experiments to study the secondary protein structure of BSA revealed varied results. The amount of β-sheet structures in BSA increased with longer stirring times in the presence of 10 μm SiNPs. To gain a better picture of the protein–nanoparticle interaction dynamics, Dr. Umezawa and team turned their attention to CD. Using the Beta Structure Selection (BeStSel) technique, which could specifically detect β-sheet-derived peaks, they found that the α-helical structure of BSA was disrupted by interaction with SiNPs. While the α-helix structure percentage in BSA decreased during interaction with SiNPs, parallel β-sheet protein confirmation was increasingly favored.
In summary, this study reveals the impact of ultra-small NPs on biological macromolecules, like proteins. The insights gained from the protein–nanoparticle interaction can guide the development of safe and effective nanoparticle-based systems for applications in various fields of medical biology.
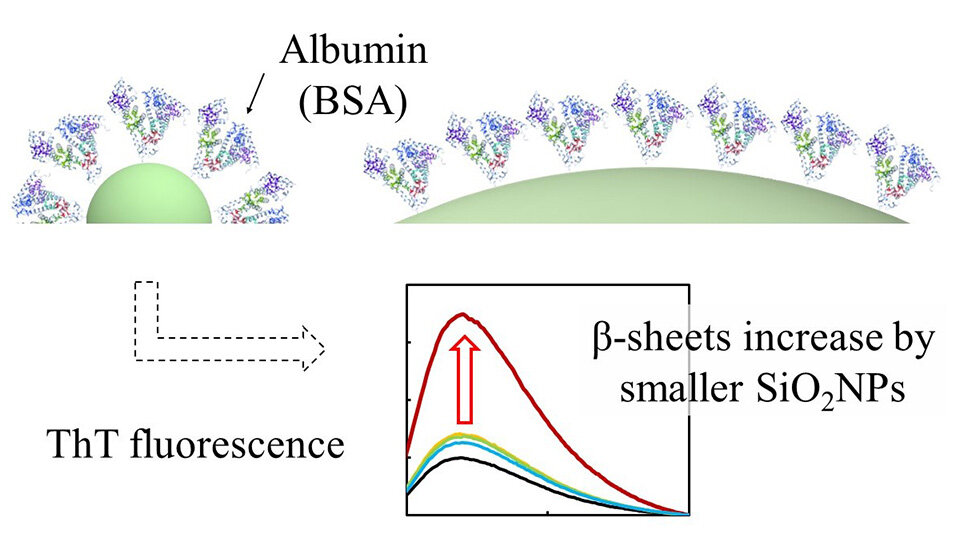
Image title: Interaction of bovine serum albumin (BSA) with silica nanoparticles
Image caption: Results of thioflavin T fluorescence analyses revealed a drastic increase in the fluorescence intensity when BSA was mixed with ultra-small (10 nm) silica nanoparticles (NPs) with a stirring time of one hour. Ultra-small NPs can induce abnormal changes in protein structure and have the potential to cause pathological conditions like Alzheimer's disease.
Image credit: Dr. Masakazu Umezawa from Tokyo University of Science, Japan
Image Source Link: https://pubs.acs.org/doi/10.1021/acs.langmuir.5c01606
License type: CC-BY 4.0
Usage restrictions: Credit must be given to the creator.
Reference
| Title of original paper | : | Changes in the Protein Secondary Structure on the Surface of Silica Nanoparticles with Different Sizes |
| Journal | : | Langmuir |
| DOI | : | 10.1021/acs.langmuir.5c01606 |
About The Tokyo University of Science
Tokyo University of Science (TUS) is a well-known and respected university, and the largest science-specialized private research university in Japan, with four campuses in central Tokyo and its suburbs and in Hokkaido. Established in 1881, the university has continually contributed to Japan's development in science through inculcating the love for science in researchers, technicians, and educators.
With a mission of "Creating science and technology for the harmonious development of nature, human beings, and society," TUS has undertaken a wide range of research from basic to applied science. TUS has embraced a multidisciplinary approach to research and undertaken intensive study in some of today's most vital fields. TUS is a meritocracy where the best in science is recognized and nurtured. It is the only private university in Japan that has produced a Nobel Prize winner and the only private university in Asia to produce Nobel Prize winners within the natural sciences field.
■
Tokyo University of Science(About TUS)

About Associate Professor Masakazu Umezawa
from Tokyo University of Science
Dr. Masakazu Umezawa serves as an Associate Professor in the Department of Medical and Robotic Engineering Design, Faculty of Advanced Engineering, Tokyo University of Science, Japan. Over the years, he has published 169 papers in high-impact factor scientific journals. His main research interests include nanomaterials chemistry, protein–nanomaterial interaction, and environmental physiology. He has won several awards and honors, such as the "Most Cited Paper Award" and "Hot Article Award 2022" in recent years. He has been associated with multiple academic societies, including the Japan Society of Bioimaging and the Japanese Biochemical Society
Laboratory website 
Official TUS website 
Funding information
This work was supported by JST SPRING (Grant Number JPMJSP2151), JST FOREST Program (Grant Number JPMJFR225B), JSPS KAKENHI (Grant Numbers 23K18403 and 22H03335), and The Kato Memorial Bioscience Foundation.

