2024.12.11 Wednesday
Researchers Harness Copper Versatility to Enable Precise Control of CO₂ Reduction Products
By deliberately engineering defects into the nanoclusters, they could direct reactions to produce methane, highlighting how structural design influences product selectivity.
Rising CO2 emissions are accelerating global warming and climate change. But what if scientists could repurpose excess CO2 into a potential energy source?
Electrochemical reduction is a promising way to achieve this. Through this catalyst-driven process, CO2 is converted into products like carbon monoxide (CO), methane (CH4), ethanol (C2H6O), or formic acid (HCOOH). Yet, barriers remain in trying to achieve industrial-scale production of specific products. This is because in CO2 reduction, the reaction can lead to several potential outcomes. Scientists, therefore, are experimenting with ways to influence the reaction pathways, making it more likely that specific products will be formed.
A group of researchers from Tohoku University, the Tokyo University of Science in Japan, and Vanderbilt University have turned to the versatile metal copper, using it as a catalyst for electrochemical CO2 reduction, to achieve controllable product specificity. By controlling the structural architecture of copper at the nanoscale, they could precisely shape the copper into nanoclusters under 2 nm in diameter, thereby enhancing the efficiency of its use as a catalyst.
Details of their research were published in Small Science on November 28, 2024.
"We explored defect-induced copper nanoclusters as a cost-effective alternative to noble metal nanoclusters, tailoring them to produce specific high-energy-density products," says Yuichi Negishi, a professor at Tohoku University's Institute of Multidisciplinary Research for Advanced Materials (IMRAM).
The team enhanced the nanoclusters' performance by creating specific active sites through deliberate defects in the cubic copper structure. By slightly dislocating some copper atoms, they prevented surface-protecting ligands from attaching to certain areas, leaving these spots exposed. These dislocated atoms appeared not only at the cube's corners but also along its edges, forming a network of reactive sites ideal for CO2 reduction. This unique arrangement of copper atoms allowed the team to guide the reaction more effectively, improving the selectivity and efficiency of the desired products.
Tests showed that nanoclusters with a single modified vertex were highly selective for producing methanol (CH3OH). However, as the number of defect sites increased, the selectivity shifted toward different products.
"Our research underscores the potential of copper nanoclusters as an affordable CO2 reduction catalyst, highlighting how their structural design influences product selectivity," adds Negishi.
Such advancements could drive the development of new functional materials from readily available resources, potentially creating a more sustainable future.
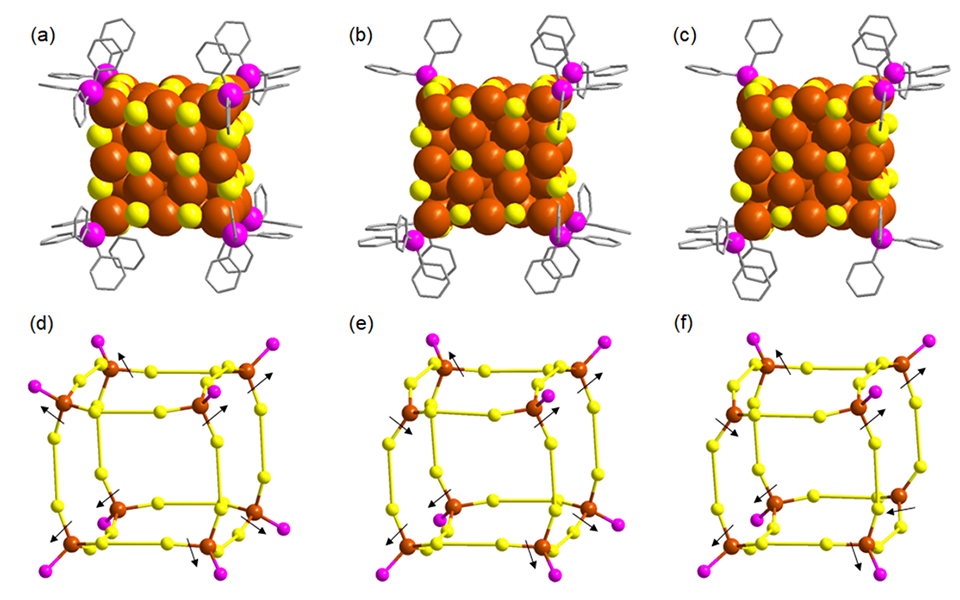
Caption: Detailed architecture of different copper nanoclusters. All the nanoclusters have identical number of copper atoms, thiolate ligands and hydrides. The alteration is visible only in PPh3 ligand attachment. (a) and (d) where all the PPh3 ligands were attached, (b) and (e) where one vertex is dislocated which influence the detachment of one PPh3 ligand, (c) and (f) where two diagonal vertices are dislocated that detach two associated PPh3 ligands. ©Yuichi Negishi et al.
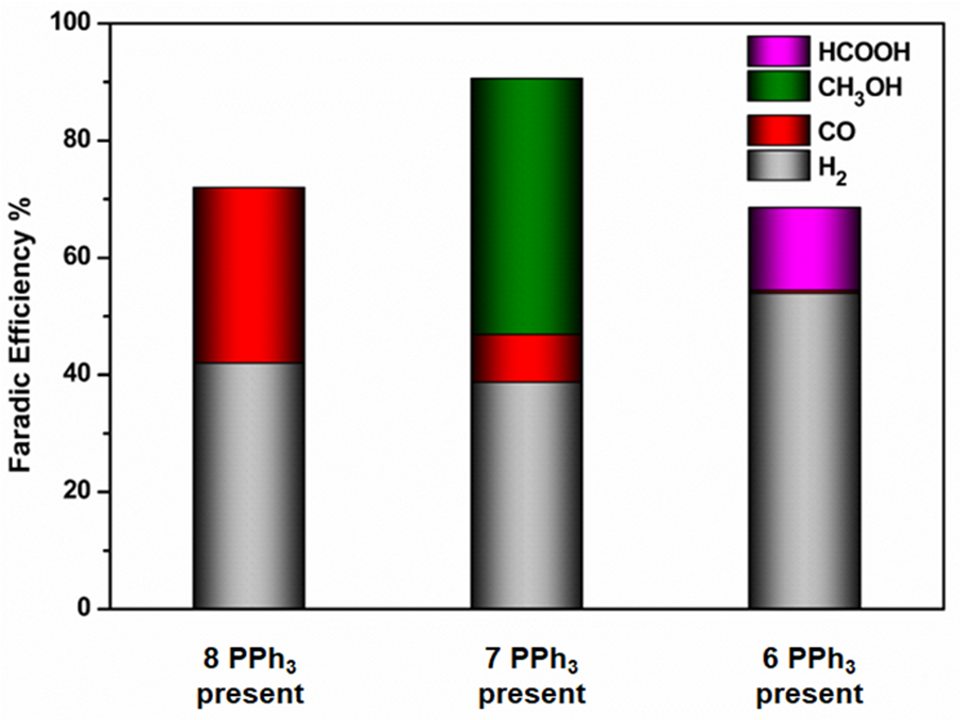
Caption: Represent the Faradic Efficiency of the CO2 reduction products of different copper nanocluster samples with different PPh3 ligand attachments. ©Yuichi Negishi et al.
Publication Details
| Title | : | Highly Selective Methanol Synthesis Using Electrochemical CO2 Reduction with Defect-Engineered Cu58 Nanoclusters |
| Authors | : | Sourav Biswas, Tomoya Tanaka, Haohong Song, Masaki Ogami, Yamato Shingyouchi, Sakiat Hossian, Maho Kamiyama, Taiga Kosaka, Riki Nakatani, Yoshiki Niihori, Saikat Das, Tokuhisa Kawawaki, De-en Jiang, Yuichi Negishi |
| Journal | : | Small Science |
| DOI | : | 10.1002/smsc.202400465 |
About The Tokyo University of Science
Tokyo University of Science (TUS) is a well-known and respected university, and the largest science-specialized private research university in Japan, with four campuses in central Tokyo and its suburbs and in Hokkaido. Established in 1881, the university has continually contributed to Japan's development in science through inculcating the love for science in researchers, technicians, and educators.
With a mission of "Creating science and technology for the harmonious development of nature, human beings, and society," TUS has undertaken a wide range of research from basic to applied science. TUS has embraced a multidisciplinary approach to research and undertaken intensive study in some of today's most vital fields. TUS is a meritocracy where the best in science is recognized and nurtured. It is the only private university in Japan that has produced a Nobel Prize winner and the only private university in Asia to produce Nobel Prize winners within the natural sciences field.
■
Tokyo University of Science(About TUS)


