2024.08.09 Friday
From Fungi to Pharmaceuticals: A Milestone for the Production of Eutyscoparol A and Violaceoid C
The approach produces naturally occurring compounds violaceoid A and eutyscoparol C with potential antimalarial and antibacterial properties
The natural world is rich in chemical compounds with remarkable medicinal properties. A notable example is penicillin, discovered by chance from the Penicillium mold. This discovery revolutionized the treatment of bacterial infections and highlighted the potential of natural compounds in medicine. Since then, the identification, isolation, and synthesis of novel bioactive compounds from plants, fungi, and bacteria have become fundamental to drug development.
Recently, two groups of naturally occurring bioactive compounds have garnered significant attention: violaceoids A–F from the fungus Aspergillus violaceofuscus and eutyscoparols A-G from the fungus Eutypella scoparia. These compounds share similar structures, featuring a 2,3-alkylated quinol moiety and a hydroxymethyl group, and are believed to possess antimalarial and antibacterial properties. Following their initial discovery in 2014 and 2020, scientists have been working to produce these compounds in larger quantities for further study.
In a recent study, researchers from Tokyo University of Science (TUS), led by Associate Professor Takatsugu Murata and Professor Isamu Shiina from the Department of Applied Chemistry, Faculty of Science, have made significant progress by developing an efficient method to synthesize eutyscoparol A and violaceoid C. Their work, featured on the cover of Volume 13, Issue 7 of the Asian Journal of Organic Chemistry, and published on 25 April 2024, could lead to new treatments or drugs.
"Eutyscoparol is a group of compounds whose pharmacological activity had not been thoroughly explored. Our goal was to make this possible through artificial synthesis and support the development of new drugs," says Dr. Murata.
The researchers used a retrosynthetic analysis to simplify the production process. This approach breaks down complex molecules into simpler, more accessible materials. They used this method to synthesize eutyscoparol A (4) and violaceoid C (3) starting from commercially available dinitriles (6) through violaceoid A (1) intermediates. Dinitriles were chosen because they are easy to obtain and can be converted into aldehydes (5), which are precursors to violaceoid A intermediates. To make the aldehyde (5), dinitrile (6) was first converted into diester. Then, the hydroxy groups in diester were protected with a tert-butyldiphenylsilyl (TBDPS) group to form protected ether. This ether was reduced to form a symmetric diol. One hydroxy group in diol was then selectively protected to create desymmetrized tetrahydropyranyl (THP)-ether, which was oxidized to produce the aldehyde.
With the aldehyde prepared, the researchers proceeded to synthesize violaceoid A (1) and rac-violaceoid B (2) intermediates through a series of reactions. To prepare violaceoid A (1), the aldehyde was first alkylated to form an intermediate, which was then converted to olefin using mesylation or the Julia–Kocienski reagent. The THP-protecting group in olefin was removed with isopropyl alcohol to produce alcohol. Finally, two TBDPS groups were removed from the alcohol to get violaceoid A (1). Rac-violaceoid B (2) was synthesized using similar methods.
These improvements made the process much more efficient. The researchers synthesized violaceoid A (1) in 8 steps with a 33% yield, compared to the previous 10-step process that had only an 11% yield. Similarly, they prepared rac-violaceoid B (rac-2) in 8 steps with a 35% yield, improving on the earlier 9-step process with a 15% yield.
After successfully synthesizing the intermediates, the researchers moved on to produce violaceoid C (3) and eutyscoparol A (4). The synthesis of violaceoid C (3) was relatively straightforward, involving the hydrogenation of the double bond in violaceoid A (1) to yield violaceoid C (3) with high efficiency. For eutyscoparol A (4), the researchers selectively methylated two of the three hydroxy groups in violaceoid A (1) by refluxing the reaction mixture with potassium carbonate and iodomethane. Overall, violaceoid C (3) was synthesized in nine steps with a 30% yield, and eutyscoparol A (4) in nine steps with a 28% yield.
With improved yields and simpler synthesis steps, the proposed approach makes it easier to produce these compounds on a larger scale and could lead to further research into their potential therapeutic properties. "The synthesis of violaceoid A and eutyscoparol C on a subgram scale will help us study their pharmacological effects, which we expect to include cytotoxic, antibacterial, and antimalarial activities," concludes Prof. Shiina.
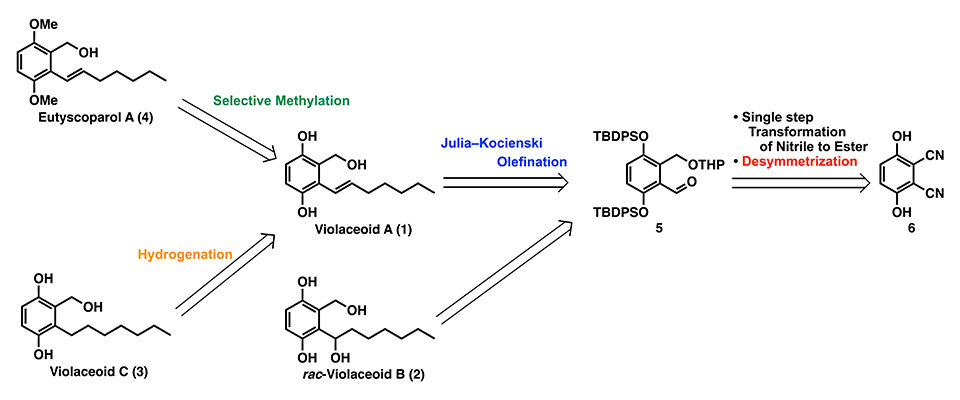
Image title: Synthesis of Eutyscoparol A (4) and Violaceoid C (3)
Image caption: The process begins with dinitriles (6), which are converted into aldehydes (5) through a series of reactions. These aldehydes serve as precursors for the synthesis of violaceoid A and B intermediates (1,2), leading to the final products, eutyscoparol A (4) and violaceoid C (3)
Image credit: Associate Professor Takatsugu Murata from TUS, Japan
License type: Original content
Usage restrictions: Not to be reproduced without permission
Reference
| Title of original paper | : | Total Synthesis of Eutyscoparol A and Violaceoid C |
| Journal | : | Asian Journal of Organic Chemistry |
| DOI | : | 10.1002/ajoc.202400148 |
About The Tokyo University of Science
Tokyo University of Science (TUS) is a well-known and respected university, and the largest science-specialized private research university in Japan, with four campuses in central Tokyo and its suburbs and in Hokkaido. Established in 1881, the university has continually contributed to Japan's development in science through inculcating the love for science in researchers, technicians, and educators.
With a mission of "Creating science and technology for the harmonious development of nature, human beings, and society," TUS has undertaken a wide range of research from basic to applied science. TUS has embraced a multidisciplinary approach to research and undertaken intensive study in some of today's most vital fields. TUS is a meritocracy where the best in science is recognized and nurtured. It is the only private university in Japan that has produced a Nobel Prize winner and the only private university in Asia to produce Nobel Prize winners within the natural sciences field.
■
Tokyo University of Science(About TUS)

About Assistant Professor Takatsugu Murata
from Tokyo University of Science
Takatsugu Murata is an Assistant Professor in the Faculty of Science Division I, Department of Applied Chemistry at Tokyo University of Science. He completed his undergraduate degree in 2014 from Tokyo University of Science and went on to earn his Master's and Doctoral degrees from the Graduate School of Chemical Sciences and Technology in 2016 and 2019, respectively. His research interest lies in synthetic chemistry, particularly in the field of organic synthetic chemistry. With a notable research background, he has authored over 17 published papers and has been granted 5 patents.
Official TUS website 
About Professor
Isamu Shiina
from Tokyo University of Science
Isamu Shiina received BSc and MSc degrees from the Tokyo University of Science in 1990 and 1992 respectively. He became an Associate Professor in 2003 and a Full Professor in 2008 at his alma mater. His research interests include the development of novel synthetic methods and the total synthesis of naturally occurring compounds. He has published over 170 papers and received numerous awards, including the Chemical Society of Japan (CSJ) Award for Young Chemists (1997), the CSJ Award for Creative Work (2013), and the Commendation for Science and Technology Prizes by the Ministries of Japan (2015).
Laboratory website 
Official TUS website 

