2024.04.16 Tuesday
Breakthrough in Benzofuran Synthesis: New Method Enables Complex Molecule Creation
Through a novel substituent migration reaction, researchers unlock a whole new world of organic molecules
In the field of organic chemistry, scientists are always looking out for new types of reactions to unlock synthesis routes for challenging compounds. Most of the progress that we have witnessed in pharmaceutics and agrochemicals over the past few decades can be traced back to the discovery of novel practical reaction pathways. Such pathways often involve the selective replacement of a functional group with another, the formation of aromatic rings, or the strategic cleaving of parts of a molecule. But what about the rearrangement of existing functional groups within a molecule?
Also known as 'substituent migration,' getting a functional group on an aromatic ring (such as in arenes) to jump to a different position in the ring is an attractive process. Chemists have come up with a few strategies to get functional groups to migrate, but the synthesis process gets significantly harder when dealing with arenes with a high number of functional groups. In particular, it is challenging to rearrange functional groups positioned next to the -OH group in phenols, a basic type of aromatic ring.
Fortunately, a research team led by Associate Professor Suguru Yoshida from Tokyo University of Science (TUS), Japan, has recently found an innovative solution to this problem. In their paper, which was published in Chemical Communications on March 28, 2024, and selected for the Themed collection "Chemical Communications HOT Articles 2024," the researchers present a new technique to synthesize various benzofurans through precise molecular rearrangement and substituent migration. Other members of the team included Dr. Akihiro Kobayashi and Mr. Shinya Tabata, both from TUS.
The researchers discovered, to their surprise, that an unusual substituent migration occurred when treating a simple aromatic compound known as o-cresol with alkynyl sulfoxide (AS) together with trifluoroacetic anhydride (TFAA). They found this reaction to partially yield a compound in which the functional group that would typically be next to the -OH group position (or 'ortho' position) was instead at the neighboring position of the aromatic ring through benzofuran ring formation. This immediately prompted them to start peering into AS/TFAA-mediated reactions further.
The team eventually figured that when a substituted phenol reacts with AS and TFFA, TFAA first activates AS, which leads to the closing of a five-membered ring sharing one of its sides with the phenol. This type of resulting molecule is called a benzofuran. Afterward, the imbalanced charges on the benzofuran trigger what's known as a 'charge-accelerated sigmatropic rearrangement.' Simply put, the formation of positively charged intermediate compounds enables the ortho functional group to migrate to the neighboring position on the phenol side.
The researchers demonstrated the versatility of their strategy by synthesizing a wide variety of benzofurans, some of which were highly functionalized or even fully functionalized. Notably, the yields of some of these compounds were exceptionally good, and in all cases, the composition of the functional groups was not damaged or altered by the process. "Our modular synthesis method enabled us to produce diverse highly substituted benzofurans from easily available starting materials through substituent migration," highlights Dr. Yoshida. "Since various benzofurans have already been used as important bioactive compounds, newly accessible benzofurans could be of great importance in pharmaceutical sciences and agrochemistry."
Overall, this study has unlocked an innovative way of simply synthesizing complex benzofurans. The researchers hope their efforts will pave the way to better anti-cancer drugs, antibiotics, fungicides, herbicides, and more. Worth mentioning, the potential applications of new highly functionalized benzofurans extend well beyond pharmaceutics and agrochemistry. They could also be used as tools in biological research, as dyes and pigments for textiles, as fragrances, and even as organic-electronic or fluorescent materials.
Now, all that is left is to perfect this promising synthesis technique and keep looking for more ways to control substituent migration. "Applications to the development of bioactive benzofurans, the synthesis of various heteroaromatics through similar reaction mechanisms, and theoretical studies with density functional theory calculations are ongoing in our laboratory," says Dr. Yoshida, with eyes on the future.
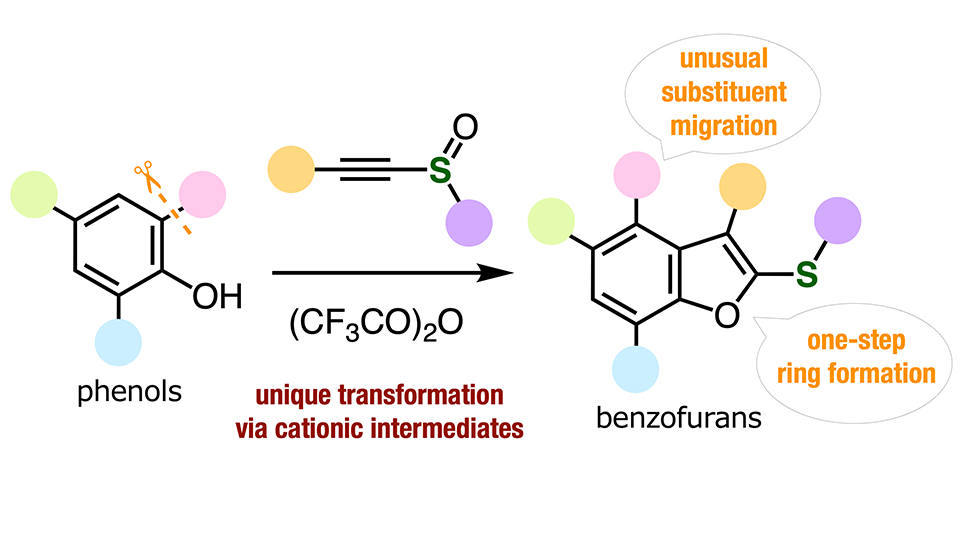
Image title: Highly functionalized benzofurans through unusual substituent migration
Image caption: This innovative synthesis strategy, which requires easily accessible starting compounds, allows for the precise formation of highly complex molecules.
Image credit: Suguru Yoshida from Tokyo University of Science
License type: Original Content
Usage restrictions: Cannot be reused without permission
Reference
| Title of original paper | : | Highly substituted benzo[b]furan synthesis through substituent migration |
| Journal | : | Chemical Communications |
| DOI | : | 10.1039/D4CC01192A |
About The Tokyo University of Science
Tokyo University of Science (TUS) is a well-known and respected university, and the largest science-specialized private research university in Japan, with four campuses in central Tokyo and its suburbs and in Hokkaido. Established in 1881, the university has continually contributed to Japan's development in science through inculcating the love for science in researchers, technicians, and educators.
With a mission of "Creating science and technology for the harmonious development of nature, human beings, and society," TUS has undertaken a wide range of research from basic to applied science. TUS has embraced a multidisciplinary approach to research and undertaken intensive study in some of today's most vital fields. TUS is a meritocracy where the best in science is recognized and nurtured. It is the only private university in Japan that has produced a Nobel Prize winner and the only private university in Asia to produce Nobel Prize winners within the natural sciences field.
■
Tokyo University of Science(About TUS)

About Associate Professor
Suguru Yoshida
from Tokyo University of Science
Dr. Suguru Yoshida is an Associate Professor at the Faculty of Advanced Engineering, Department of Biological Science and Technology, Tokyo University of Science, Japan. He received his B.S. and M.S. degrees from the University of Tokyo. After receiving his Ph.D. in engineering from Kyoto University (2009), he served as a Postdoctoral Fellow (Kyushu University and University of Hawaii at Manoa), an Associate Professor (Tokyo Medical and Dental University), and a Program Officer (MEXT), prior to his current role. He has won multiple awards, including the Thieme Chemistry Journals Award, the Young Scientists' Prize, and the Commendation for Science and Technology by MEXT. He has published over 130 articles, garnering over 3,600 citations in his primary research area of synthetic organic chemistry and chemical biology.
Laboratory website 
Official TUS website 
Funding information
This work was supported by JSPS KAKENHI (Grant Number JP22H02086), The Uehara Memorial Foundation, Tokuyama Science Foundation, The Ube Foundation, Inamori Research Grants, and JST SPRING (Grant Number JPMJSP2120).

