2024.02.15 Thursday
Scientists Achieve First Total Synthesis of Potentially Anti-Rheumatic Sesquiterpene Merillianin
The breakthrough method paves the way for the pharmaceutical synthesis of drugs for treating nervous system diseases
An avenue that scientists are currently exploring for the development of novel pharmaceuticals involves the synthesis of bioactive compounds found in Chinese herbal medicine. This collaborative effort, combining traditional knowledge with modern scientific methods, focuses on pharmaceutically relevant compounds found in medicinal plants for large-scale synthesis. An important compound in this context is merrillianin, a type of illicium sesquiterpene that was isolated in 2002 from the fruit of Illicium merrillianum, a plant that belongs to the same genus as star anise. Illicium sesquiterpenes are naturally occurring compounds which hold promise for treating nervous system diseases. However, merrillianin has a complex structure with a central arrangement of six consecutive stereogenic carbon centers, including three quaternary carbon stereogenic centers, and three rings fused to two carbons. This complexity has posed challenges for the artificial synthesis of merrillianin, leading to limited progress in its practical application since its isolation.
In a breakthrough study published in the journal Organic Letters on 31 December 2023, a research group led by Assistant Professor Takatsugu Murata and Professor Isamu Shiina from Tokyo University of Science (TUS) succeeded in synthesizing merrillianin, opening doors to its artificial synthesis almost 20 years after the compound was isolated.
"Illicium sesquiterpenes are a group of compounds that are expected to be effective against neurological diseases, but their highly oxidized and ring-fused structures have made it difficult to synthesize them artificially. However, we have synthetic technique and knowledge about the synthesis of highly complicated compounds such as taxol," says Dr. Murata. "Therefore, we wanted to perform the world's first artificial synthesis of merrillianin, which is expected to have anti-rheumatic activity, and create a lead compound that can contribute to the treatment of neurological diseases."
Merrillianin can be obtained with yields as high as 80% via the Wacker-type oxidation of a dilatone. However, the challenge lies in efficiently preparing the precursor compounds for the dilatone. To address this, the researchers employed a total of 30 reaction steps, covering the synthesis of precursors to the final production of merrillianin. The process commences with the Mukaiyama aldol reaction, which involves enol silyl ether and acetaldehyde. This reaction leads to the creation of a dithioacetal, a compound that includes a quaternary carbon stereogenic center. Subsequently, the dithioacetal undergoes a series of reactions with an iodo compound, resulting in the formation of α, β-unsaturated ester possessing an aldol structure. The next steps involve a reductive intramolecular cyclization of this compound to cyclopentane, followed by an intramolecular Michael's reaction for the formation of tricyclic dilactone with a total yield of 1.6%. Tricyclic dilactone is a key intermediate for the commercial production of a wide variety of Illicium sesquiterpene compounds, including merrillianin.
The researchers point out that if merrillianin has high bioactivity, the amount required for treatment would be very little. (According to the isolation report, 3 mg of merrillianin was isolated from 30 kg of fruit.) Interestingly, it would be possible to examine its bioactivity using the synthetic version prepared by the group.
The synthesis method also revealed the absolute configuration of merrillianin, which, so far, had only known relative configurations. The proposed synthesis method for merrillianin represents another milestone for the research group, which previously succeeded in synthesizing the naturally occurring tanzawaic acid B found in the fungus Penicillium citrinum that has the potential for developing antibiotics against multidrug-resistant bacteria.
The research group's ongoing dedication to synthesizing compounds with interesting biological activities holds promise for future discoveries in the field of drug development. Species of the Illicium genus have been used as medicinal herbs for the treatment of conditions like rheumatoid arthritis and traumatic injuries, and the synthesis of merrillianin could also contribute to advancements in these areas. "The proposed synthesis method for merrillianin will help develop suitable drugs to treat nervous system diseases such as rheumatism, and neuralgia, improving neurological disease prognosis and enhancing patient quality of life," concludes Prof. Shiina.
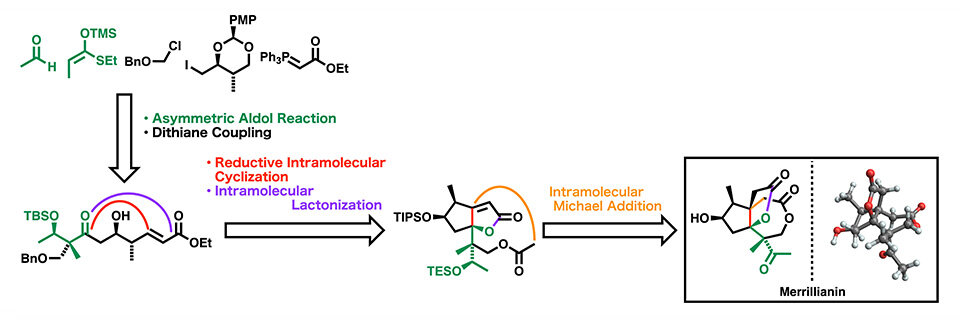
Image title: The Concept of Total Synthesis of Merrillianin
Image caption: The artificial synthesis of merrillianin opens doors to the development of drugs for treating nervous system diseases such as rheumatism and neuralgia.
Image credit: Isamu Shiina from TUS, Japan
License type: Original Content
Usage restrictions: Not to be reproduced without permission
Reference
| Title of original paper | : | Total Synthesis of the Sesquiterpene (−)-Merrillianin |
| Journal | : | Organic Letters |
| DOI | : | 10.1021/acs.orglett.3c03877 |
About The Tokyo University of Science
Tokyo University of Science (TUS) is a well-known and respected university, and the largest science-specialized private research university in Japan, with four campuses in central Tokyo and its suburbs and in Hokkaido. Established in 1881, the university has continually contributed to Japan's development in science through inculcating the love for science in researchers, technicians, and educators.
With a mission of "Creating science and technology for the harmonious development of nature, human beings, and society," TUS has undertaken a wide range of research from basic to applied science. TUS has embraced a multidisciplinary approach to research and undertaken intensive study in some of today's most vital fields. TUS is a meritocracy where the best in science is recognized and nurtured. It is the only private university in Japan that has produced a Nobel Prize winner and the only private university in Asia to produce Nobel Prize winners within the natural sciences field.
■
Tokyo University of Science(About TUS)

About Professor Isamu Shiina from Tokyo University of Science
Isamu Shiina received BSc and MSc degrees from the Tokyo University of Science in 1990 and 1992 respectively. He became an Associate Professor in 2003 and a Full Professor in 2008 at his alma mater. His research interests include the development of novel synthetic methods and the total synthesis of naturally occurring compounds. He has published over 170 papers and received numerous awards, including the Chemical Society of Japan (CSJ) Award for Young Chemists (1997), the CSJ Award for Creative Work (2013), and the Commendation for Science and Technology Prizes by the Ministries of Japan (2015).
Laboratory website 
Official TUS website 
About Assistant Professor Takatsugu Murata from Tokyo University of Science
Takatsugu Murata is an Assistant Professor in the Faculty of Science Division I, Department of Applied Chemistry at Tokyo University of Science. He completed his undergraduate degree in 2014 from Tokyo University of Science and went on to earn his Master's and Doctoral degrees from the Graduate School of Chemical Sciences and Technology in 2016 and 2019, respectively. His research interest lies in synthetic chemistry, particularly in the field of organic synthetic chemistry. With a notable research background, he has authored 13 published papers and has been granted a patent.
Official TUS website 

