2024.01.29 Monday
New Horizons in Chemical Biology: A Novel Approach to Synthesize Dibenzothiophene S-Oxides
The approach enables the synthesis of dibenzothiophene S-oxides with highly reactive functional groups, surpassing conventional methods

Organic compounds in the field of chemistry range from simple hydrocarbons to complex molecules, with diverse functional groups added to the main carbon backbone. These functional groups impart the compounds distinct chemical properties as well as participate in various chemical transformations, making them important precursors for the synthesis of diverse compounds. Scientists have, therefore, actively engaged in creating molecules that feature novel and highly reactive functional groups.
One such class of compounds are dibenzothiophenes and their derivatives containing S-oxide or S,S-dioxide moieties (sulfur atoms bonded to one and two oxygen atoms respectively). These compounds are of special interest in the fields of pharmaceutical sciences, materials chemistry, and chemical biology. Dibenzothiophenes consist of benzene rings fused to a thiophene ring―a five-membered ring with four carbon atoms and one sulfur atom. When dibenzothiophene S-oxides are exposed to UV light, they release atomic oxygen, which is useful for DNA cleavage and oxidation of adenosine-S'-phosphosulfate kinase, an enzyme involved in cellular processes. Additionally, the S-O bond can be activated to introduce different functional groups, enabling the creation of a wide range of molecules with diverse properties and applications. The conventional method of producing functionalized dibenzothiophene S-oxides involves thiophene ring formation followed by subsequent S-oxidation. However, this reaction is challenging to carry out.
To address this, Associate Professor Suguru Yoshida, Ms. Yukiko Kumagai, Mr. Akihiro Kobayashi, and Mr. Keisuke Nakamura from Tokyo University of Science (TUS) have developed a simple two-step method of synthesizing dibenzothiophene S-oxides. The method involves Suzuki-Miyaura coupling of 2-bromoaryl-substituted sulfinate esters, followed by an intramolecular electrophilic sulfinylation.
The details of the method, published in the journal Chemical Communications on 10 January 2024, opens possibilities for creating a variety of important sulfur-containing molecules in the life sciences that were traditionally difficult to synthesize using conventional methods.
"Dibenzothiophene oxides are attracting attention in the field of chemical biology, and several researchers have developed a reaction using dibenzothiophene oxide, which can now be synthesized using this method. We expect this research to elucidate life phenomena involving reactive oxygen species," explains Dr. Yoshida, while talking about this study.
The Suzuki-Miyaura coupling is a widely used organic reaction between boronic acids and organic halides, leading to the formation of a new carbon-carbon bond. In the proposed method, sulfinate esters react first with arylboronic acids in the presence of a palladium catalyst. Next, the intermediate biaryl compounds are activated with Tf2O, leading to subsequent cyclization by electrophilic activation.
Compared to the conventional oxidation method of synthesizing dibenzothiophene, this innovative approach developed by Dr. Yoshida and his team can accommodate a wide range of functional groups, including highly reactive ones, enabling the synthesis of polysubstituted dibenzothiophene oxides not achievable earlier. Using the method, the researchers synthesized dibenzothiophene oxides having an o-silylaryl triflate moiety, a compound useful as an aryne generation site, but tends to get easily damaged when produced using conventional methods. The o-silylaryl triflate moiety serves as a useful reactive intermediate and can undergo various transformations to produce highly substituted arenes. The proposed method, therefore, not only simplifies the synthesis method but also opens doors for a diverse range of dibenzothiophene S-oxides and their derivatives.
The novel method is a significant step forward in the field of chemical biology. Going ahead, the researchers anticipate that these compounds can find useful applications in diverse research areas, paving the way for innovations and discoveries. "The proposed method can enable the synthesis of polysubstituted benzothiophene oxides, which are expected to be useful in a wide range of research fields," concludes Dr. Yoshida.
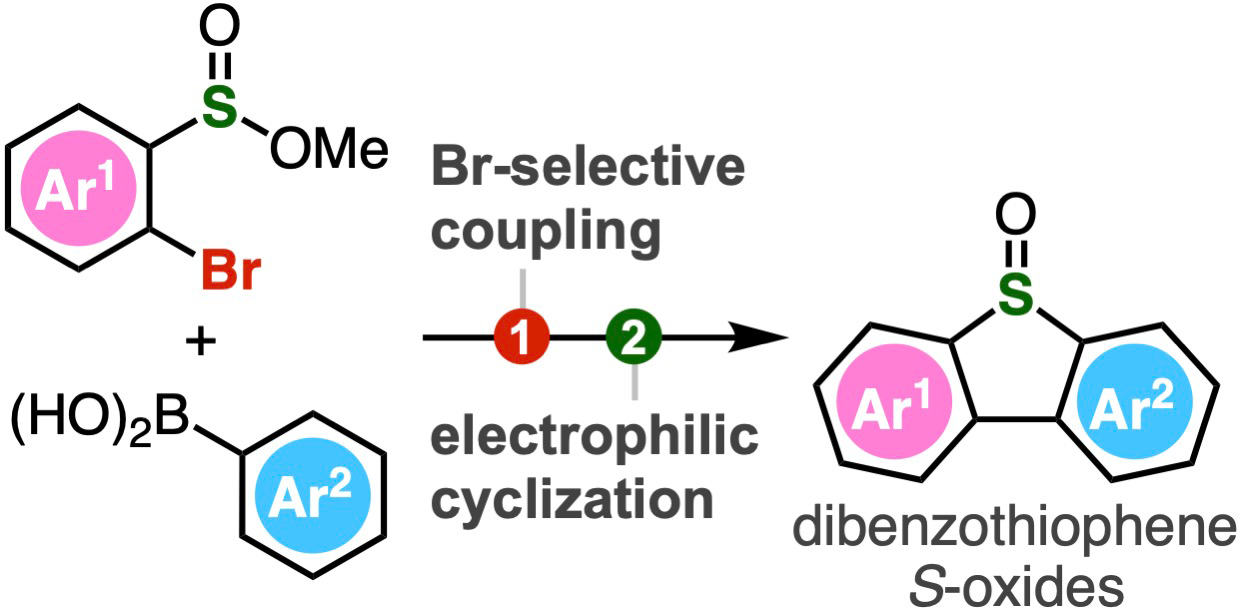
Image title: Schematic of the two-step process for producing dibenzothiophene S-oxides
Image caption: A range of polysubstituted dibenzothiophene oxides can be synthesized through Br-selective coupling and subsequent cyclization by electrophilic activation.
Image source link: https://pubs.rsc.org/en/content/articlelanding/2024/cc/d3cc05703h 
Image credit: Suguru Yoshida from Tokyo University of Science
Usage restrictions: Credit must be given to the creator. Only noncommercial uses of the work are permitted.
License type: CC BY-NC 3.0
Reference
| Title of original paper | : | Facile Synthesis of Dibenzothiophene S-Oxides from Sulfinate Esters |
| Journal | : | Chemical Communications |
| DOI | : | 10.1039/D3CC05703H |
About The Tokyo University of Science
Tokyo University of Science (TUS) is a well-known and respected university, and the largest science-specialized private research university in Japan, with four campuses in central Tokyo and its suburbs and in Hokkaido. Established in 1881, the university has continually contributed to Japan's development in science through inculcating the love for science in researchers, technicians, and educators.
With a mission of "Creating science and technology for the harmonious development of nature, human beings, and society," TUS has undertaken a wide range of research from basic to applied science. TUS has embraced a multidisciplinary approach to research and undertaken intensive study in some of today's most vital fields. TUS is a meritocracy where the best in science is recognized and nurtured. It is the only private university in Japan that has produced a Nobel Prize winner and the only private university in Asia to produce Nobel Prize winners within the natural sciences field.
■
Tokyo University of Science(About TUS)

About Associate Professor
Suguru Yoshida
from Tokyo University of Science
Dr. Suguru Yoshida is an Associate Professor at the Faculty of Advanced Engineering, Department of Biological Science and Technology, Tokyo University of Science, Japan. He received his B.S. and M.S. degrees from the University of Tokyo. After receiving his Ph.D. in engineering from Kyoto University (2009), he served as a Postdoctoral Fellow (Kyushu University and University of Hawaii at Manoa), an Associate Professor (Tokyo Medical and Dental University), and a Program Officer (MEXT), prior to his current role. He has won multiple awards, including the Thieme Chemistry Journals Award, the Young Scientists' Prize, and the Commendation for Science and Technology by MEXT. He has published over 130 articles, garnering over 3,600 citations in his primary research area of synthetic organic chemistry and chemical biology.
Laboratory website 
Official TUS website 
Funding information
This work was supported by JSPS KAKENHI Grant Number JP22H02086 (S. Y.); The Uehara Memorial Foundation (S. Y.); Tokuyama Science Foundation (S. Y.); The Ube Foundation (S. Y.); and Inamori Research Grants (S. Y.)

