2023.10.16 Monday
Novel Enzyme Family Could Provide Insights into Bacterial Pathogenicity
Researchers discover a new family of Gram-negative bacterial enzymes related to infection capability

Gram-negative bacteria cause a variety of infectious diseases in plants and animals alike. Outbreaks of Salmonella and E. coli infections often make headlines due to their severity, and people have to resort to allopathic as well as natural remedies, increasing the burden on the healthcare system. While antibiotics offer an effective solution against bacterial infections, the increasing incidence of antibiotic-resistant bacteria have prompted researchers to identify other possible treatments against these infections. With technological advances and modern medicine, researchers are looking into the possibility of disrupting the pathogenicity of the bacteria at a molecular level by interfering with molecular processes at the gene as well as protein level.
Gram-negative bacteria, notorious for their infection capability, produce osmo-regulated periplasmic glucans (OPGs)―long-chain carbohydrates made of multiple glucose units―in the extracellular and/or periplasmic space. Initially, it was believed that OPGs were by-products produced under low solute concentrations, but recent reports confirm that they are crucial for pathogenicity, symbiosis, cell adhesion, and signaling.
However, the enzymes involved in the synthesis, regulation, and degradation of OPGs are not fully known. Genetic analysis revealed that the removal of opgH and/or opgG genes, partially responsible for OPG synthesis, causes bacteria to lose their infection capability, suggesting strong potential links of these genes with bacterial pathogenicity.
Although the structure of OpgG from E. coli (EcOpgG) has been elucidated, the mechanism of action of OpgG and OpgD from E. coli (EcOpgG and EcOpgD, respectively) remains unclear. Understanding the enzymes involved in OPG synthesis and the mechanisms underlying their function could provide us vital insights into the pathogenicity of Gram-negative bacteria, allowing us to develop more effective ways to deal with bacterial infections.
To bridge this gap in knowledge, Mr. Sei Motouchi from Tokyo University of Science, Dr. Kaito Kobayashi from the National Institute of Advanced Industrial Science and Technology (AIST), Associate, Associate Professor Hiroyuki Nakai from Niigata University and Professor Masahiro Nakajima from the Tokyo University of Science conducted structural and functional analyses of EcOpgD and EcOpgG. The study was published in Communications Biology on September 21, 2023.
Sharing the motivation behind this study, Professor Nakajima tells us, "Glycans are important biological macromolecules that play a variety of roles in living organisms, including pathogenicity and symbiosis. Their structure is very diverse and complex, and thus there are many types of enzymes that may synthesize and degrade them. However, we humans know only a small fraction of them".
The researchers investigated the functions of OPG-related genes in the model organism E. coli. Functional analyses revealed that E. coli OpgD (EcOpgD) was an endo-β-1,2-glucanase, which specifically broke down β-1,2-glucans. It also had similar kinetic properties as those of general glycoside hydrolases (GH), further confirming its identity as a β-1,2-glucanase.
Structural analysis using crystallography revealed a high degree of similarity between the structures of EcOpgG and EcOpgD. However, the two enzymes had remarkably different activity. Upon further investigation, the researchers found that a few amino acids forming the reaction pathway, termed 'Loop A', were critical for enzyme activity and regulated the rate of reaction. EcOpgG and EcOpgD differed in their catalytic functions, possibly due to the difference in the amino acids in the Loop A region. The LoopA region diversifies among this group of enzymes, which may lead to functional diversity. Nevertheless, the basis of the catalytic center is shared in this group of enzymes. This common point will help scientists develop therapies that could potentially disrupt OPG synthesis and hinder the infection capability of bacteria.
Further, while the two enzymes belonged to the same family of GHs, their structure did not match with any of the existing GH enzymes. Thus, the authors confirmed that they belonged to a novel GH family, namely GH186. This information opens avenues for research into therapies that can target GH186 proteins to stop the progression of bacterial infections.
Professor Masahiro concludes by explaining the long-term applications of the study, "Although it was known that some Gram-negative plant pathogens synthesize OPGs for pathogenicity, most of the key enzymes for their synthesis had not been identified, preventing the development of agrochemicals targeting OPGs. We have identified a family of enzymes (GH186) involved in the direct synthesis of OPGs and elucidated their detailed functions, which has presented us with new targets (GH186) to inhibit pathogens and provides a solid foundation for 'structure-based pesticide discovery'".
The findings of this study lay down a strong foundation for further investigation of OPGs and related genes and may usher in a new era of disease management.
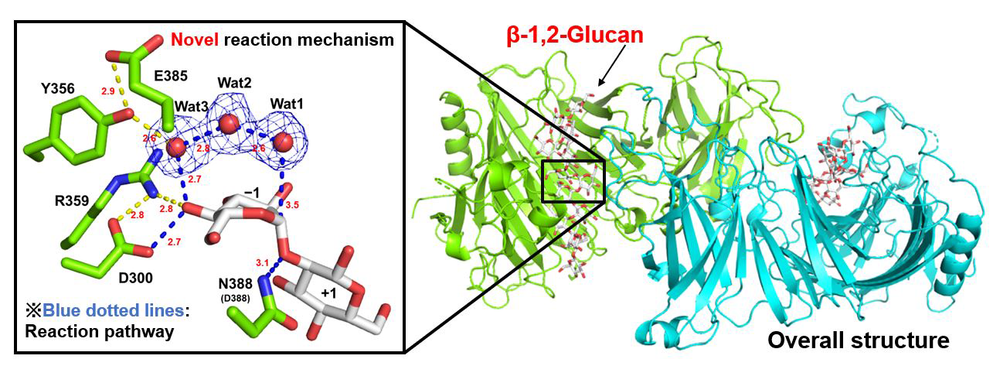
Title: 3D structure of EcOpgD with β-1,2-glucan obtained by X-ray crystallography
Caption: EcOpgD is a β-1,2-glucanase involved in the adjustment of the chain length of osmo-regulated periplasmic glucans (OPGs). A reaction mechanism with an unprecedentedly long proton transfer pathway has been proposed for EcOpgD.
Image credits: Masahiro Nakajima, Tokyo University of Science
License type: Original Content
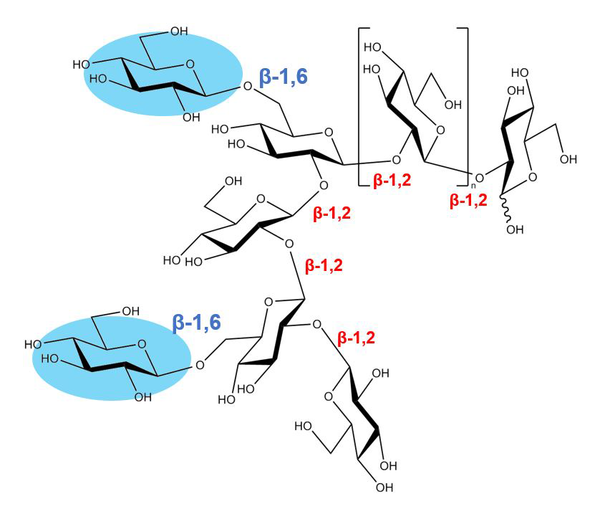
Title: Osmo-regulated periplasmic glucans found in Escherichia coli
Caption: Osmo-regulated periplasmic glucans (OPGs) are known to be vital for conferring pathogenicity to Gram-negative bacteria. The main chain of OPGs is β-1,2-linked glucooligosaccharide. The detailed positions of β-1,6-glucose side chains in this diagram were not elucidated.
Image credits: Masahiro Nakajima, Tokyo University of Science
License type: Original Content
Reference
| Title of original paper | : | Identification of enzymatic functions of osmo-regulated periplasmic glucan biosynthesis proteins from Escherichia coli reveals a novel glycoside hydrolase family |
| Journal | : | Communications Biology |
| DOI | : | 10.1038/s42003-023-05336-6 |
| Authors | : | Sei Motouchi1, Kaito Kobayashi2, Hiroyuki Nakai3 and Masahiro Nakajima1 |
| Affiliations | : |
1Department of Applied Biological Science, Faculty of Science and Technology, Tokyo University of Science 2Artificial Intelligence Research Center, National Institute of Advanced Industrial Science and Technology (AIST) 3Faculty of Agriculture, Niigata University |
Further Information
Associate Professor Masahiro Nakajima
Department of Applied Biological Science, Faculty of Science and Technology
Tokyo University of Science
Email: m-nakajima(at sign)rs.tus.ac.jp
Associate Professor Hiroyuki Nakai
Faculty of Agriculture
Niigata University
Email: nakai(at sign)agr.niigata-u.ac.jp
Media contact
Hiroshi Matsuda
Public Relations Divisions
Tokyo University of Science
Email: mediaoffice(at sign)admin.tus.ac.jp
Website 
Public Relations office
Niigata University
Email: pr-office(at sign)adm.niigata-u.ac.jp
About The Tokyo University of Science
Tokyo University of Science (TUS) is a well-known and respected university, and the largest science-specialized private research university in Japan, with four campuses in central Tokyo and its suburbs and in Hokkaido. Established in 1881, the university has continually contributed to Japan's development in science through inculcating the love for science in researchers, technicians, and educators.
With a mission of "Creating science and technology for the harmonious development of nature, human beings, and society," TUS has undertaken a wide range of research from basic to applied science. TUS has embraced a multidisciplinary approach to research and undertaken intensive study in some of today's most vital fields. TUS is a meritocracy where the best in science is recognized and nurtured. It is the only private university in Japan that has produced a Nobel Prize winner and the only private university in Asia to produce Nobel Prize winners within the natural sciences field.
■
Tokyo University of Science(About TUS)

About Professor Masahiro Nakajima
from Tokyo University of Science
Funding information
This work was supported in part by JST SPRING, Grant Number JPMJSP2151.

