2023.09.07 Thursday
Extending the Playing Field for Organosulfurs: A New Way to Synthesize Sulfinate Esters
Scientists develop a novel synthesis route to produce a wide variety of sulfinate esters using readily available compounds

Organosulfur compounds are organic molecules that contain one or more sulfur atoms bonded to carbon atoms. They not only play fundamental roles in biological processes but also have wide applications in many industries, such as pharmaceuticals, agrochemicals, and materials science. Thus, many chemists strive to develop safe and efficient methods to synthesize organosulfurs.
The conventional approach to produce them involves the oxidation of molecules called thiols. However, working with thiols can be quite challenging. They have a strong and unpleasant odor and can be oxidized easily under air, which makes handling and storage difficult. These two issues have limited the availability of thiols with interesting functional groups, also hindering the production of different types of organosulfurs. But what if we could produce organosulfurs from less problematic chemicals?
In a recent study published in Organic and Biomolecular Chemistry on 11 August 2023, a research team from Japan has come up with a new approach to synthesize sulfinate esters, a subclass of organosulfur compounds, using thioesters. The research, led by Associate Professor Suguru Yoshida, is co-authored by Mr. Keisuke Nakamura, Ms. Yukiko Kumagai, Mr. Akihiro Kobayashi, and Ms. Minori Suzuki, all from Tokyo University of Science (TUS).
Thioesters have essentially the same chemical structure as esters, except that one or two oxygen atoms are replaced by sulfur atoms. Unlike thiols, thioesters are odorless, stable, and easily accessible, which makes them easier to work with. These advantages motivated the research team to develop an efficient synthesis route for the synthesis of sulfinate esters via direct oxidation of thioesters.
They first prepared a desired thioester molecule from an aryl iodide composed of an aryl group bound to an iodine atom. Using a copper-containing catalyst, the researchers managed to strip the iodine atom from the aryl group and replace it with a carbon-sulfur bond, forming a thioester. Afterwards, the thioester was directly oxidized in the presence of N-bromosuccinimide, which created an intricate reaction pathway culminating with the formation of a sulfinate ester.
This two-step synthesis technique is efficient and straightforward. Most importantly, it carries the potential to produce various sulfinate esters from easily available starting materials, including carboxylic acids, anilines, and a wide variety of aryl iodides. "Compared to conventional preparation methods of sulfinate esters from other sulfur surrogates, the superior accessibility of aryl iodides from a wide variety of aromatic compounds will enable the synthesis of highly functionalized sulfinate esters," remarks Dr. Yoshida.
Overall, the method proposed in this study will greatly bolster research on new organosulfurs, leading to promising applications in many fields. For example, sulfinate esters are used in the synthesis of sulfonamide-containing compounds, which have antimicrobial, anti-inflammatory, and enzyme inhibitory activities. They are also used to produce drugs with sulfoxide groups, which can have various biological activities, including anti-clotting and anti-acid effects. Moreover, sulfinate esters can help synthesize functional polymers and agrochemicals and serve as reagents in analytical chemistry techniques to detect the presence of specific compounds or functional groups.
With eyes on the future, Dr. Yoshida concludes: "Further studies towards finding applications for the preparation of bioactive organosulfur derivatives, as well as the synthesis of bis-sulfinate esters, are underway in our laboratory."
Let us hope that this study opens up new possibilities for organosulfurs.
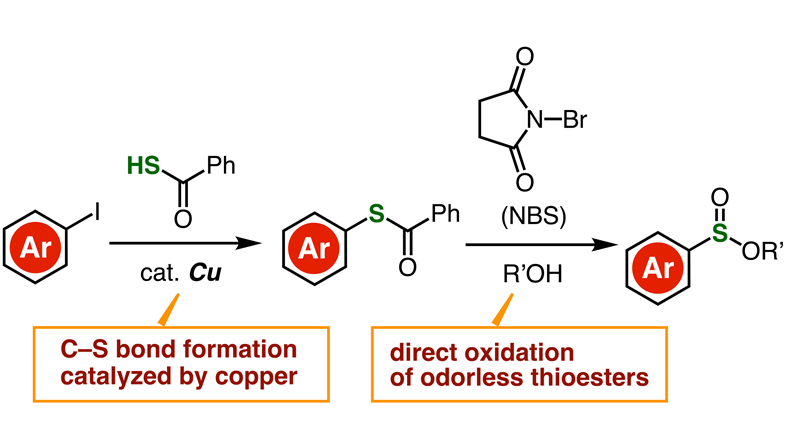
Image title: Producing sulfinate esters from aryl iodides
Image caption: Schematic of the proposed two-step synthesis route to easily produce a wide variety of sulfinate esters. The use of aryl iodides, which are readily available, is a remarkable advantage.
Image credit: Suguru Yoshida from Tokyo University of Science
Image source link: https://pubs.rsc.org/en/content/articlelanding/2023/OB/D3OB01108A
License type: CC BY-NC 3.0
Reference
| Title of original paper | : | Facile synthesis of sulfinate esters from aryl iodides via direct oxidation of thioesters |
| Journal | : | Organic and Biomolecular Chemistry |
| DOI | : | 10.1039/D3OB01108A |
About The Tokyo University of Science
Tokyo University of Science (TUS) is a well-known and respected university, and the largest science-specialized private research university in Japan, with four campuses in central Tokyo and its suburbs and in Hokkaido. Established in 1881, the university has continually contributed to Japan's development in science through inculcating the love for science in researchers, technicians, and educators.
With a mission of "Creating science and technology for the harmonious development of nature, human beings, and society", TUS has undertaken a wide range of research from basic to applied science. TUS has embraced a multidisciplinary approach to research and undertaken intensive study in some of today's most vital fields. TUS is a meritocracy where the best in science is recognized and nurtured. It is the only private university in Japan that has produced a Nobel Prize winner and the only private university in Asia to produce Nobel Prize winners within the natural sciences field.
■
Tokyo University of Science(About TUS)

About Associate Professor Suguru Yoshida
from Tokyo University of Science
Dr. Suguru Yoshida is an Associate Professor at the Faculty of Advanced Engineering, Department of Biological Science and Technology, Tokyo University of Science, Japan. He received his B.S. and M.S. degrees from the University of Tokyo. After receiving his Ph.D. in engineering from Kyoto University (2009), he served as a Postdoctoral Fellow (Kyushu University and University of Hawaii at Manoa), an Associate Professor (Tokyo Medical and Dental University), and a Program Officer (MEXT) prior to his current role. He has won multiple awards, including Thieme Chemistry Journals Award, the Young Scientists' Prize, and the Commendation for Science and Technology by MEXT. He has published over 130 articles, garnering over 3,600 citations in his primary research area of synthetic organic chemistry and chemical biology.
Laboratory website 
Official TUS website 
Funding information
This work was supported by JSPS KAKENHI Grant Number JP22H02086, The Uehara Memorial Foundation, Tokuyama Science Foundation, The Ube Foundation, and Inamori Research Grants.

