2023.05.25 Thursday
New Study Shows Superior Reactive Oxygen Species Removal Ability of Copper Coupled to Lysozyme
Researchers develop a novel hybrid protein complex by binding lysozyme to copper for enhanced reactive oxygen species removal
Cu(II) complexes with low molecular weight are functional superoxide-dismutase (SOD) models with superior reactive oxygen species (ROS) removal ability. However, concerns regarding their toxicity after the release of Cu(II) limits their use for ROS removal. Now, researchers from Japan have designed a novel lysozyme coupled Cu(II) hybrid protein complex that demonstrates enhanced SOD activity and excellent stability. Such unique hybrid biocompatible metal-protein SOD complexes may be viable low-toxicity alternatives in therapeutic drug applications.
In aerobic organisms, reactive oxygen species (ROS), such as hydroxide (OH), singlet oxygen (1O2), hydrogen peroxide (H2O2), and superoxide (O2-) ions are produced during aerobic respiration, which causes serious oxidative damage to biomolecules in the body. Hence, the removal of ROS, particularly O2-, is of primary importance as it reacts with H+ to produce other toxic ROS species like H2O2 and OH.
This is achieved by metalloenzymes called superoxide dismutases (SODs). These enzymes possess metal ions (like Ni, Fe, Mn, Cu, and Zn) in their active centers that catalyze the decomposition of O2- to H2O2 and O2. In this regard, low molecular weight Cu(II) complexes have gained importance as functional SOD models that exhibit high SOD activity. However, they are limited by their tendency to become toxic to the biomolecules after the release of Cu(II).
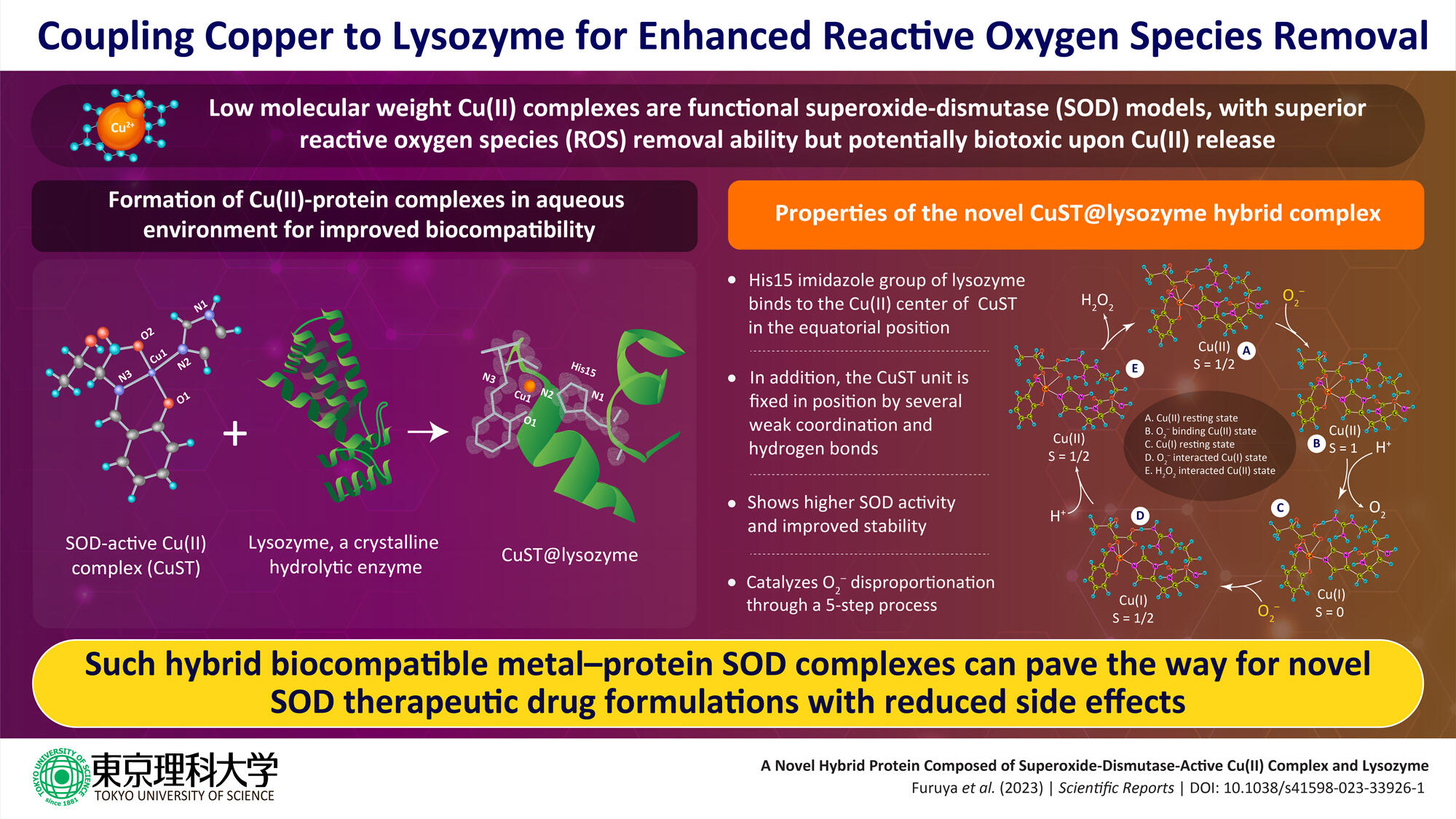
In a recent study, a group of researchers led by Assistant Professor Daisuke Nakane and Professor Takashiro Akitsu from the Department of Chemistry, Tokyo University of Science (TUS), has developed a novel metal-protein hybrid complex with enhanced ROS activity. They coupled the hydrolytic enzyme lysozyme with SOD-active Cu(II) complex to form the hybrid lysozyme CuST@lysozyme, which showed promising SOD activity but low biotoxicity.
"We investigated the formation of a hybrid protein composed of lysozyme and a functional SOD-mimetic Cu(II) complex. We chose lysozyme owing to its stability and crystallinity. We theorized that the resulting SOD-mimetic hybrid protein would improve the biocompatibility and stability of the functional SOD model Cu(II) complex," explains Dr. Nakane as the rationale behind their study. The study was published on 27 April 2023 in Scientific Reports.
Through detailed crystallographic and spectroscopic analysis, the research team, which also comprised Assistant Professor Kenichi Kitanishi from TUS, Dr. Arshak Tsaturyan from Southern Federal University, and Professor Masaki Unno from Ibaraki University, among others, confirmed the formation of the hybrid protein CuST@lysozyme, and elucidated its structure. They report that the His15 imidazole group of the lysozyme binds to the Cu(II) center of CuST in the equatorial position while the CuST unit is fixed axially by several weak coordination and hydrogen bonds. Further they also suggest that O2- can coordinate to the Cu(II) center. Through assays, the researchers established high SOD activity and stability of the biocompatible CuST@lysozyme hybrid protein complex.
Based on their spectroscopic and quantum calculations, the team propose a five-step mechanism of O2- disproportionation by the complex. These steps are (1) Cu(II) resting state, (2) O2--binding Cu(II) state, (3) Cu(I) resting state after protonation of the carboxylate ligand, (4) O2--interacted Cu(I) state, and (5) H2O2-interacted Cu(II) state. They further suggest that the stability of the complex can be improved by suppressing ligand dissociation by using late-transition-metal complexes for binding lysozyme, increasing interaction between the complexes and lysozyme by using ligands with hydrogen-bonding moieties, and introducing acidic functional groups to counter the basic side chains of lysozyme.
The study introduces a new class of SOD active hybrid protein complexes that are biocompatible and have no side reactions with bodily fluids after decomposition of the mimetic complex. "We have strategically improved the stability of the metal-lysozyme composites, specifically in biological fluids such as plasma and cytosol. This should pave the way for deeper discussions on their therapeutic applications," concludes Prof. Akitsu.
We will certainly benefit from advancements like these that add to our repertoire of biocompatible complexes for advanced therapeutics.
Reference
| Title of original paper | : | A Novel Hybrid Protein Composed of Superoxide‑Dismutase‑Active Cu(II) Complex and Lysozyme |
| Journal | : | Scientific reports |
| DOI | : | 10.1038/s41598-023-33926-1 |
About The Tokyo University of Science
Tokyo University of Science (TUS) is a well-known and respected university, and the largest science-specialized private research university in Japan, with four campuses in central Tokyo and its suburbs and in Hokkaido. Established in 1881, the university has continually contributed to Japan's development in science through inculcating the love for science in researchers, technicians, and educators.
With a mission of "Creating science and technology for the harmonious development of nature, human beings, and society", TUS has undertaken a wide range of research from basic to applied science. TUS has embraced a multidisciplinary approach to research and undertaken intensive study in some of today's most vital fields. TUS is a meritocracy where the best in science is recognized and nurtured. It is the only private university in Japan that has produced a Nobel Prize winner and the only private university in Asia to produce Nobel Prize winners within the natural sciences field.
■
Tokyo University of Science(About TUS)

About Assistant Professor Daisuke Nakane
from Tokyo University of Science
Dr. Daisuke Nakane is an Assistant Professor at the Faculty of Science Division, Department of Chemistry, Tokyo University of Science, Japan. He graduated from the Department of Applied Chemistry, Nagoya Institute of Technology in 2005 and obtained his Ph.D. in Material Engineering in 2010. He was then awarded the Vice President's Commendation (Academic Research Activity Division). His research interests include inorganic chemistry, coordination chemistry, and bioinorganic chemistry.
Official TUS website

About Professor Takashiro Akitsu
from Tokyo University of Science
Dr. Takashiro Akitsu is a Professor in the Department of Chemistry, Faculty of Science, Tokyo University of Science (TUS), Japan. He graduated from Osaka University and obtained his Ph.D. in Physical and Inorganic Chemistry in 2000 and went on to study physical and bioinorganic chemistry at Stanford before moving to TUS. He joined the TUS as a Junior Associate Professor in 2008 and became a Professor in 2016. His current research areas involve the study of imines, Schiff bases, coordination chemistry, and crystal structures.
Laboratory website

Official TUS website

Funding information
This study was partially supported by a Grant-in-Aid for Scientific Research (A) KAKENHI (20H00336) and Ministry of Science and Higher Education of the Russian Federation No. 0852-2020-0019 (state assignment in the field of scientific activity, Southern Federal University, 2020 project No BAZ0110/20-1-03EH).

