2023.04.10 Monday
Study Unravels Pathophysiological Role of Dectin-1 in Promoting Colorectal Cancer
The team led by Tokyo University of Science researchers identified the mechanism using mouse models and validated it with clinical samples
Colorectal cancer is associated with significant mortality. However, the precise mechanism of action that governs the development of colorectal cancer remains largely unknown. A research team led by scientists from the Tokyo University of Science has recently been able to show that the receptor protein "Dectin-1" promotes colorectal tumorigenesis by enhancing the production of prostaglandin E2, which in turn suppresses the expression of the tumor-inhibitory IL-22-binding protein.
Colorectal cancer (CRC) causes nearly 500,000 deaths every year across the globe. Although CRC is predominantly associated with old age and poor dietary habits, the precise pathophysiological mechanisms that contribute to the development of CRC continue to remain elusive. Now, a research team―led by Professor Yoichiro Iwakura from Tokyo University of Science, Japan, and Professor Ce Tang from Sun Yat-sen University China―has recently been able to identify the underlying mechanism using a mouse model and clinical samples. Their results have been published in Nature Communications. This paper was published online in Volume 14 Issue 1 of the journal on March 17, 2023.
"We investigated the role of Dectin-1 in colorectal tumorigenesis by analyzing mouse intestinal tumor models and clinical samples from patients with CRC. We showed that Dectin-1 signaling promotes the development of colorectal tumors by enhancing the production of prostaglandin E2 (PGE2), which facilitates CRC development by suppressing the expression of the tumor-inhibitory IL-22-binding protein (IL-22BP)," says Prof. Iwakura.
Dectin-1 primarily serves as a receptor protein and preferentially binds to β-glucans―glucose polymers that naturally occur in the cell walls of various types of fungi. Although prior studies have shown that Dectin-1 offers protection against fungal invasion, the current study highlights its role as a receptor protein involved in the development of CRC.
To fully understand the underlying mechanism of Dectin-1's pathophysiological action in CRC, the research team generated genetically modified "Clec7a−/− mice" that were deficient in Dectin-1. For this purpose, the team used the ApcMin mouse model of human familial adenomatous polyposis a form of cancer characterized by multiple tumors―as well as the azoxymethane (AOM)-dextran sodium sulfate (DSS)-induced colorectal cancer model of chemical carcinogenesis. Quite interestingly, Clec7a−/− mice showed reduced tumorigenesis in both of the above models, thus underscoring the role of Dectin-1 in CRC development.
Next, the researchers decided to investigate the role of gut bacteria in intestinal tumorigenesis. To this end, they created germ-free (GF) mice that harbored no commensal bacteria in their guts. They found that, in the complete absence of any gut bacteria, colorectal polyp number in Clec7a−/− GF mice was greatly reduced compaired with wild type GF mice, showing that gut microbiota are not involved in the reduction of polyps in Clec7a−/− mice.
The team then decided to delve into the associated mechanism of action. Subsequent murine-model-based experiments revealed that PGE2 levels in tumors were reduced in Clec7a−/− mice. Moreover, they also observed a reduction in the expression of PGE2 synthases such as COX2 which is known to promote intestinal tumorigenesis.
Furthermore, while investigating the types of cells that produced PGE2 synthases, the researchers found that it is mainly produced by myeloid cell-derived suppressor cells (MDSCs) that have infiltrated into the colorectal tumor. In addition, the researchers also demonstrated that PGE2 promoted the differentiation and proliferation of MDSCs, further contributing to the development of CRC in the murine models.
While attempting to elucidate the underlying mechanism of action, the researchers also noticed that Clec7a−/− mice showed an increased production of IL-22BP―a protein that can suppress the development of colorectal tumors by binding and inhibiting the pro-inflammatory protein Interleukin-22 (IL-22). Deletion of the gene responsible for the expression of IL-22BP caused increased polyps and early death in ApcMin mice, thus underscoring the role of IL-22BP in tumor suppression. Moreover, the production of IL-22BP was found to be strongly suppressed by PGE2.
Interestingly, laminarin, a low-molecular-weight β-glucan from seaweeds, significantly inhibited AOM-DSS-induced colonic tumorigenesis in mice that were fed with this compound. The team also found that whereas high-molecular-weight β-glucans promoted tumor growth, low-molecular-weight β-glucans suppressed it, by suppressing Dectin-1 signaling.
These results also have immediate clinical implications. For instance, the team noticed that patients with CRC showing low CLEC7A expression survived longer than those with high expression of CLEC7A (in the MDSCs). Moreover, in patients with CRC, IL22RA2 expression was decreased and that of PTGS2―a PGE2-synthesizing enzyme―was increased in tumors compared to in normal tissues.
Prof. Iwakura concludes, "Dectin-1 plays a key role in the development of colorectal tumorigenesis in both mice and humans, through the modification of PGE2 and IL-22BP levels. Dectin-1, therefore, serves as an attractive target for the development of novel anti-CRC therapeutics."
These findings are groundbreaking and make a significant contribution toward our present understanding of the genesis of colorectal cancer. Further research along this direction will be sure to aid in the prevention and treatment of this high-mortality disease.
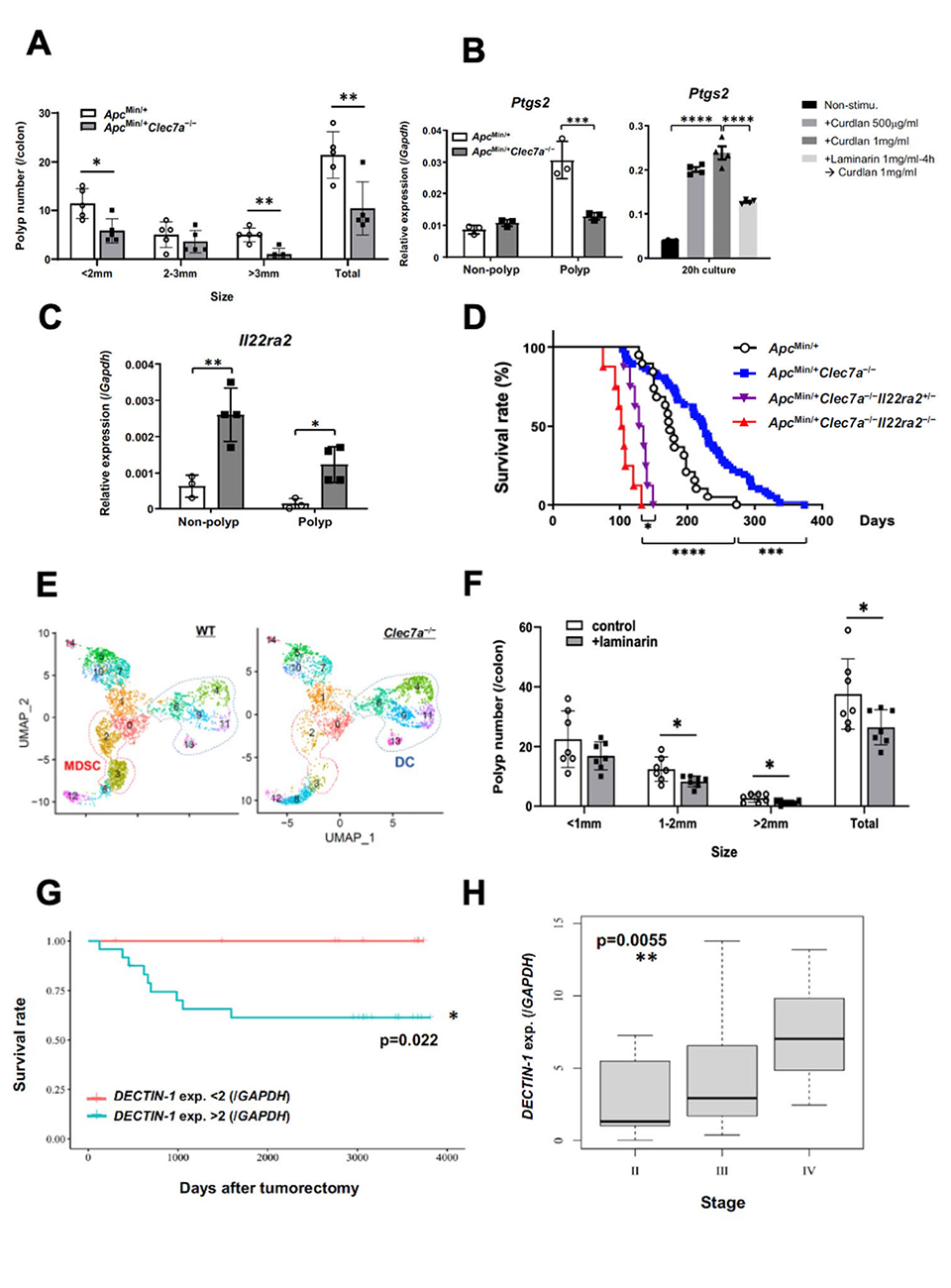
Image title: Delineating the pathophysiological role of Dectin-1 in colorectal tumorigenesis based on murine and clinical studies
Image caption: A. Mouse model of familial colorectal adenomatosis (ApcMin) showing significantly fewer colonic polyps in Dectin-1-deficient (Clec7a−/−) mice; B. Polyps show reduced expression of PGE2 synthase (Cox2). In vitro, Cox2 expression is induced in myeloid cells by curdlan―a high-molecular-weight β-glucan―and conversely inhibited by laminarin―a low-molecular-weight β-glucan; C. Increased IL-22BP expression in Clec7a−/− mice; D. Dectin-1 gene loss increases the lifespan of ApcMin mice, whereas deletion of the IL-22BP gene results in a shorter lifespan; E. The number of myeloid-derived suppressor cells (MDSCs) is reduced in Clec7a−/− mice; F. Dietary laminarin reduces the number of AOM-DSS-induced colon polyps G. Among colorectal cancer patients, those with high dectin-1 expression have significantly lower survival rates than those with low dectin-1 expression; H. Dectin-1 expression in colorectal cancer patients correlates with cancer severity.
Image credit: Yoichiro Iwakura from Tokyo University of Science
Image Source Link to be added in the Image Credit Section of EA form:
https://www.nature.com/articles/s41467-023-37229-x
License type: CC BY 4.0
Reference
| Title of original paper | : | Blocking Dectin-1 prevents colorectal tumorigenesis by suppressing prostaglandin E2 production in myeloid-derived suppressor cells and enhancing IL-22 binding protein expression |
| Journal | : | Nature Communications |
| DOI | : | 10.1038/s41467-023-37229-x |
About The Tokyo University of Science
Tokyo University of Science (TUS) is a well-known and respected university, and the largest science-specialized private research university in Japan, with four campuses in central Tokyo and its suburbs and in Hokkaido. Established in 1881, the university has continually contributed to Japan's development in science through inculcating the love for science in researchers, technicians, and educators.
With a mission of "Creating science and technology for the harmonious development of nature, human beings, and society," TUS has undertaken a wide range of research from basic to applied science. TUS has embraced a multidisciplinary approach to research and undertaken intensive study in some of today's most vital fields. TUS is a meritocracy where the best in science is recognized and nurtured. It is the only private university in Japan that has produced a Nobel Prize winner and the only private university in Asia to produce Nobel Prize winners within the natural sciences field.
■
Tokyo University of Science(About TUS)

About Professor Prof. Yoichiro Iwakura
from Tokyo University of Science
Prof. Yoichiro Iwakura obtained his Ph.D. from the Kyoto University and is currently serving as a Professor at the Tokyo University of Science. He has over 690 peer-reviewed articles and more than 25 patents to his credit. Prof. Iwakura's research group primarily focuses on autoimmune diseases and pathophysiological mechanisms, as well as on the development of health-promoting foods and novel therapeutics. Prof. Iwakura has won several accolades for his pioneering research work and is a highly cited biomedical researcher with publications in Nature Immunology, Cell, and Immunity.
Laboratory website

Funding information
This study was supported by General Program of National Natural Science Foundation of China (82070564, C.T.), the Foundation of 100 Talents Program of Sun Yat-Sen University (Y61231, C.T.), the Fundamental Research Funds for the Central Universities, Sun Yat-sen University (C.T.), the Grants-in Aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan [Scientific Research (B), (20H03176, C.T. & 18H02671, Y.I.), and JSPS KAKENHI Grant-in-Aid for Scientific Research on Innovative Areas 18H05045 and 2R.(Y.I.)], and was carried out as a collaboration research with Boehringer Ingelheim International GmbH (Y.I.).

