2023.04.06 Thursday
New Study Reveals Design Clues for Silver-based Superatomic Molecules
Researchers from Japan explore the key factors responsible for the synthesis of materials composed of silver-based superatoms
Superatomic molecules containing noble metal elements like gold and silver are studied for their potential in the synthesis of superatomic materials. However, the understanding of silver-based superatomic molecules has been limited. Addressing this gap, researchers from Japan studied two bimetallic superatomic molecules with silver as a main constituent to determine the key factors that enabled their formation. Their findings are expected to advance the development of novel materials in the future.
In the past few decades, metal nanoclusters composed of noble metal elements such as gold (Au) and silver (Ag) have gained attention as superatoms for the synthesis of materials with unique properties and potential new applications. These superatoms (also known as "artificial atoms") typically consist of a cluster of a few to several hundred atoms and exhibit properties that are significantly different from their bulk, conventional counterparts. However, much like real atoms, the stability of these superatoms is determined by the formation of a closed-shell electron structure.
Ag-based superatoms are known for their superior properties and functions, including photoluminescence and selective catalytic activity, compared to those of Au-based superatoms. However, most of the research in this field has been primarily focused on Au-based superatomic molecules.
To overcome this research gap, researchers from Japan studied the formation of superatomic molecules composed of Ag and evaluated the factors involved in this formation. This study was led by Professor Yuichi Negishi from Tokyo University of Science (TUS) with contributions from Dr. Sakiat Hossain from TUS, and Professor Tetsuya Taketsugu and Assistant Professor Takeshi Iwasa from Hokkaido University, and was published in the journal Communications Chemistry on 28 March 2023.
Speaking of the motivation behind studying Ag-based superatoms, Prof. Negishi says, "So far, we humans have created a variety of useful materials from the elements available to us on earth. However, looking at a future with complex energy and environmental issues, the development of materials with new properties and functions is desired."
To this end, the researchers synthesized two di-superatomic molecules with bromine (Br) as the bridging ligand: ([Ag23Pt2(PPh3)10Br7]0 and [Ag23Pd2(PPh3)10Br7]0 (PPh3 = Triphenylphosphine). The former consisted of two icosahedral Ag12Pt superatoms connected by vertex sharing with platinum atoms (Pt) occupying the central position in each superatom. In contrast, the other superatomic molecule consisted of two icosahedral Ag12Pd structures with palladium (Pd) as the central atom.
The geometric/electronic structure and stability of these two nanoclusters was then analyzed and compared with [Ag23Pt2(PPh3)10Cl7]0 (1) and [Ag23Pd2(PPh3)10Cl7]0 (2) - two nanoclusters with geometrical similarity to the synthesized nanoclusters, consisting of chlorine (Cl) as the bridging atom.
On examining the geometric structures of the four nanoclusters, the researchers observed a twist between the two icosahedral structures containing Br as the bridging ligand. The researchers suggest that this twist stabilizes the nanocluster by shortening the distance between the two icosahedral structures.
Additionally, the larger Br atom was found to introduce steric hindrance in the molecule, causing both the PPh3 molecule to be positioned further from the long axis of the metal nanocluster and, a change in the bond length of the Ag-P and Ag-Ag bonds. These findings indicate that although the type of bridging halogen slightly affects the geometric structures of the metal nanoclusters, it does not hinder their formation.
"The type of bridging halogen appears to have little effect on whether superatomic molecules can be formed or not, as long as the bridging halogen is large enough to maintain a moderate distance between the two Ag12M structures," explains Prof. Negishi.
However, the stability of the nanocluster was largely dependent on the number of bridging halogens attached to it. Like atoms, stable metallic nanoclusters require a filled valence shell. In the case of the prepared nanoclusters ― which had a total of 16 valence electrons ― the researchers were only able to attach a maximum of five bridging halogens to maintain the metal nanocluster in a stable neutral or cationic state.
The presence of Pd and Pt central atoms was found to be due to the formation of metallic nanoclusters. Substituting the central atom of Ag13 with Pt or Pd led to an increase in the average binding energy within the nanoclusters, making it favorable for the formation of superatomic molecules.
Overall, the researchers identified three key requirements for the formation and isolation of superatomic molecules consisting of two Ag13−xMx structures connected by vertex sharing. These include the presence of a bridging halogen that can maintain an optimal distance between the two structures, a combination of heteroatoms and bridging halogens that results in 16 valence electrons, and the formation of an icosahedral core that is stronger than Ag13.
In the words of Prof. Negishi, "These findings offer clear design guidelines for the creation of molecular devices with various properties and functions, and can potentially contribute to resolving pressing concerns regarding clean energy and the environment."
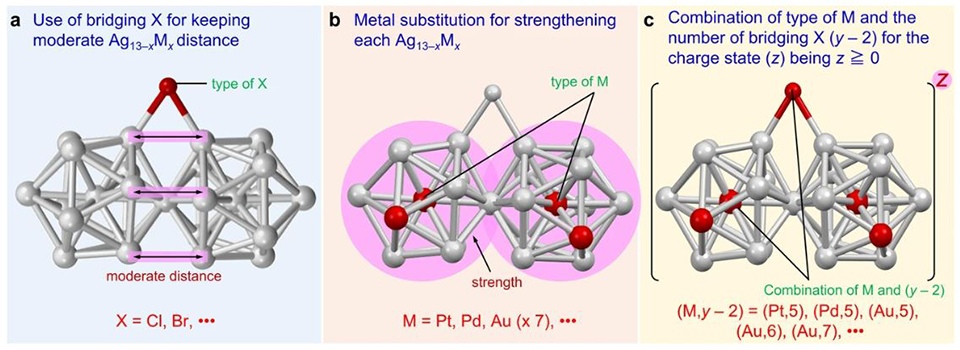
Image title: The three important requirements for the formation and isolation of Ag13−xMx structures connected by vertex sharing.
Image caption: The formation of stable Ag-based nanoclusters require: (a) a bridging halogen that can maintain a moderate distance between the two Ag13−xMx structures, (b) a metal atom for a stable icosahedral core and (c) a combination of heteroatoms and bridging halogens that results in 16 valence electrons.
Image credit: Yuichi Negishi from TUS, Japan
License type: CC BY 4.0
Reference
| Title of original paper | : | Key factors for connecting silver-based icosahedral superatoms by vertex sharing |
| Journal | : | Communications Chemistry |
| DOI | : | 10.1038/s42004-023-00854-0 |
| Authors | : | Sayuri Miyajima1, Sakiat Hossain2, Ayaka Ikeda1, Taiga Kosaka1, Tokuhisa Kawawaki1,2, Yoshiki Niihori2, Takeshi Iwasa3,4, Tetsuya Taketsugu3,4, and Yuichi Negishi1,2 |
| Affiliations | : |
1 Department of Applied Chemistry, Faculty of Science, Tokyo University of Science 2 Research Institute for Science & Technology, Tokyo University of Science 3 Department of Chemistry, Faculty of Science, Hokkaido University 4 WPI-ICReDD, Hokkaido University |
Further information
Professor Yuichi Negishi
Department of Applied Chemistry, Faculty of Science
Tokyo University of Science
Email: negishi【@】rs.tus.ac.jp
Assistant Professor Takeshi Iwasa
Department of Chemistry, Faculty of Science
Hokkaido University
Email: tiwasa【@】sci.hokudai.ac.jp
Media contact
Hiroshi Matsuda
Public Relations Division
Tokyo University of Science
Email: mediaoffice【@】admin.tus.ac.jp
Website 
Sohail Keegan Pinto
Public Relations & Communications Division
Office of Public Relations and Social Collaboration
Hokkaido University
Email: en-press【@】general.hokudai.ac.jp
About The Tokyo University of Science
Tokyo University of Science (TUS) is a well-known and respected university, and the largest science-specialized private research university in Japan, with four campuses in central Tokyo and its suburbs and in Hokkaido. Established in 1881, the university has continually contributed to Japan's development in science through inculcating the love for science in researchers, technicians, and educators.
With a mission of "Creating science and technology for the harmonious development of nature, human beings, and society," TUS has undertaken a wide range of research from basic to applied science. TUS has embraced a multidisciplinary approach to research and undertaken intensive study in some of today's most vital fields. TUS is a meritocracy where the best in science is recognized and nurtured. It is the only private university in Japan that has produced a Nobel Prize winner and the only private university in Asia to produce Nobel Prize winners within the natural sciences field.
■
Tokyo University of Science(About TUS)

About Professor Yuichi Negishi
from Tokyo University of Science
About Assistant Professor Sakiat Hossain
from Tokyo University of Science
Funding information
This study was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI (grant numbers 20H02698 and 20H02552), Scientific Research on Innovative Areas "Innovations for Light-Energy Conversion" (grant numbers 18H05178 and 20H05115), Scientific Research on Innovative Areas "Hydrogenomics" (grant number 21H00027), and Scientific Research on Innovative Areas "Aquatic Functional Materials" (grant numbers and 22H04562). Funding provided by the Yazaki Memorial Foundation for Science and Technology, the Ogasawara Foundation for the Promotion of Science and Engineering, and TEPCO Memorial Foundation, the Japan Science Society, the Takahashi Industrial and Economic Research Foundation, and the Kubota Corporation is also gratefully acknowledged.

