2022.07.18 Monday
Novel Multi-Proton Carrier Complex as Efficient Proton Conductor at High Temperatures
Researchers develop a highly symmetric ruthenium (III) complex with six imidazole-imidazolate groups for efficient high-temperature proton conduction in fuel cells
Fuel cells often fall short when it comes to operating at temperatures beyond 100 ֯C owing to their dependence on water as a proton conduction medium. To overcome this issue, a team of researchers from Japan designed a new hydrogen-bonded starburst-shaped metal complex consisting of ruthenium (III) ion and six imidazole-imidazolate groups. The resulting single molecular crystal shows excellent proton conductivity even at temperatures as high as 180°C and as low as -70 °C.
As the world is moving towards more environment-friendly and sustainable sources of energy, fuel cells are receiving a lot of attention. The main advantage of fuel cells is that they use hydrogen, a clean fuel, and produce only water as a by-product while generating electricity. This new and clean source of electricity could replace conventional lithium-ion batteries, which currently power all modern electronic devices.
Most fuel cells use a Nafion membrane― a synthetic polymer-based ionic membrane― which serve as a water-based proton conducting solid electrolyte. The use of water as a proton conduction medium, however, creates a major drawback for the fuel cell, namely the inability to function properly at temperatures above 100 ֯ C, the temperature at which water starts to boil, leading to a drop in proton conductivity. Therefore, there is a need for new proton conductors that can transfer protons efficiently even at such high temperatures.
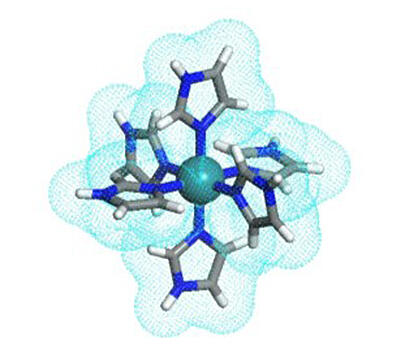
In a recent breakthrough, a team of researchers from Japan, led by Prof. Makoto Tadokoro from Tokyo University of Science (TUS), reported a novel imidazole-imidazolate metal complex based high-temperature proton conductor that shows efficient proton conductivity even at 147°C (Fig.1). The research team included Dr. Fumiya Kobayashi from TUS, Dr. Tomoyuki Akutagawa and Dr. Norihisa Hoshino from Tohoku University, Dr. Hajime Kamebuchi from Nihon University, Dr. Motohiro Mizuno from Kanazawa University, and Dr. Jun Miyazaki from Tokyo Denki University." Imidazole, a nitrogen-containing organic compound, has gained popularity as an alternative proton conductor for its ability to operate even without water. However, it has a lower proton transfer rate than conventionally used Nafion and melts at 120°C. To overcome these issues, we introduced six imidazole moieties into a ruthenium (III) ion to design a new metal complex that operates as a multi-proton carrier and has high temperature stability," explains Prof. Tadokoro when asked about the rationale behind their study, which was published online first on June 27, 2022 in Chemistry - A European Journal and featured on the front cover of the journal.
The team designed a new molecule where three imidazole (HIm) and three imidazolate (Im-) groups were attached to a central ruthenium (III) ion (Ru3+). The resulting single molecular crystal was highly symmetrical and resembled a "starburst" shape. Upon investigating the proton conductivity of this starburst-type metal complex, the team found that each of the six imidazole groups attached to the Ru3+ ion acts as a proton transmitter. This made the molecule 6 times more potent than individual HIm molecules, which could only transport one proton at a time.
The team also explored the mechanism underlying the high-temperature proton conduction ability of the starburst molecules. They found that at a temperature of more than -70°C, the proton conductivity resulted from individual localized rotations of the HIm and Im- groups and proton jump to other Ru(III) complexes in the crystal via hydrogen bonds. At temperatures beyond 147°C, however, the proton conductivity arose from whole-molecule rotation, which was also responsible for the superior proton conductivity at high temperatures. This rotation, confirmed by the team using a technique called "solid-state 2H-NMR spectroscopy," resulted in a conductivity rate three orders of magnitude larger (σ = 3.08 × 10-5 S/cm) than that for individual HIm molecules (σ = 10-8 S/cm).
The team believes that their study could act as a new driving principle for proton-conducting solid-state electrolytes. The insights from their novel molecular design could be used to develop new high-temperature proton conductors as well as improve the functionality of the existing ones. " Fuel cells hold the key to a cleaner and greener tomorrow. Our study offers a roadmap for improving the performance of these carbon-neutral energy resources at high temperatures by designing and implementing molecular proton conductors that can transfer protons efficiently at such temperatures, " concludes Prof. Tadokoro.
Hopefully, we're not too far from realizing a future based on fuel cells and clean energy.
Reference
| Title of original paper | : | Proton Conduction at High Temperature in High-Symmetry Hydrogen-Bonded Molecular Crystal of Ru(III) Complexes with Six Imidazole-Imidazolate Ligands |
| Journal | : | Chemistry - A European Journal |
| DOI | : | 10.1002/chem.202201397 |
About The Tokyo University of Science
Tokyo University of Science (TUS) is a well-known and respected university, and the largest science-specialized private research university in Japan, with four campuses in central Tokyo and its suburbs and in Hokkaido. Established in 1881, the university has continually contributed to Japan's development in science through inculcating the love for science in researchers, technicians, and educators.
With a mission of "Creating science and technology for the harmonious development of nature, human beings, and society", TUS has undertaken a wide range of research from basic to applied science. TUS has embraced a multidisciplinary approach to research and undertaken intensive study in some of today's most vital fields. TUS is a meritocracy where the best in science is recognized and nurtured. It is the only private university in Japan that has produced a Nobel Prize winner and the only private university in Asia to produce Nobel Prize winners within the natural sciences field.



About Professor Makoto Tadokoro from Tokyo University of Science
Dr. Makoto Tadokoro is a Professor at the Faculty of Science Division I, Department of Chemistry at Tokyo University of Science (TUS), Japan. An inorganic chemist and materials scientist, he obtained his Ph.D. degree from Kyushu University, Japan, in 1992. He then went on to work at the Institute of Molecular Science as a Research Associate before he joined as an Associate professor at Osaka University in 1995. He joined TUS in 2005. His current areas of research include supramolecular coordination chemistry, water cluster science, molecular conductors, and protonics. He has over 160 published papers to his name, with about 2100 citations to his credit. He is also affiliated with various leading organizations in the field, such as the Chemical Society of Japan, and has won several awards for his work.
https://www.tus.ac.jp/en/fac/p/index.php?4870 
https://www.rs.kagu.tus.ac.jp/tadokoro/
Funding information
This study was funded by a Grant-in-Aid for Scientific Research (No. 15H03851) in Priority Areas from the Ministry of Education, Science and Culture, Japan.
|
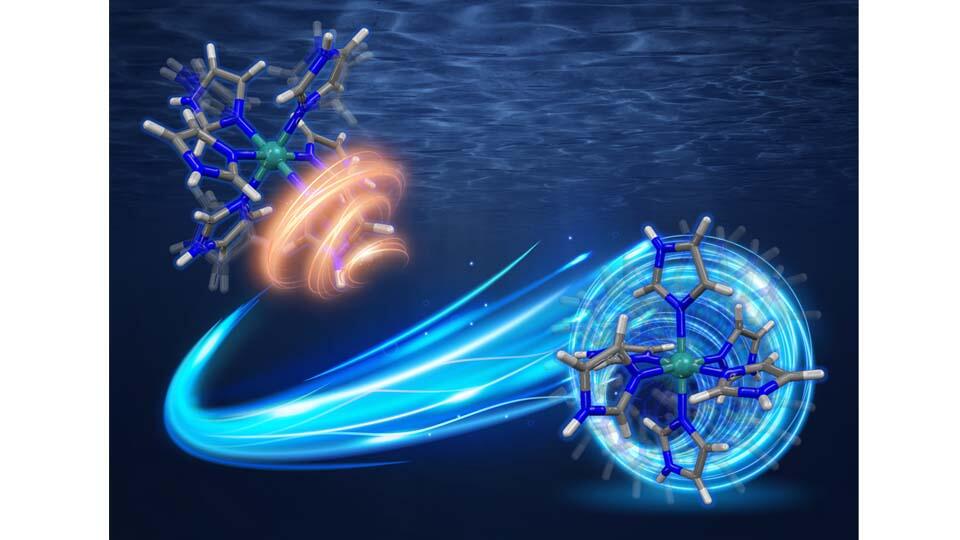
Title: Two rotation modes of ruthenium (III) complexes with six imidazole-imidazolate ligands.
Caption: In a new study, researchers from Japan have developed a novel ruthenium (III) ion complex with six imidazole/imidazolate groups that can operate as multi-proton carriers and shows high temperature stability. The top image depicts the proton transport mode below 147˚C, which involves individual localized rotations of the six individual imidazole groups and proton jumps to other ruthenium (III) complexes. The bottom image depicts the proton transport mode above 147˚C, where the whole molecule undergoes rotation.
Image Credit: Makoto Tadokoro from Tokyo University of Science
License type: under CC-BY license
Image link: https://chemistry-europe.onlinelibrary.wiley.com/doi/10.1002/chem.202201397

