2022.07.14 Thursday
Changes in Protein Structure and Assembly with Fluoride Nanoparticles and Coexisting Ions
Scientists demonstrate the influence of nanoparticles and surrounding ions on the formation of β-sheet structure in amyloid β proteins
Protein function and activity is determined by both their assembly and secondary structure. Abnormalities related to either protein aggregation or secondary structure can lead to neurodegenerative diseases. In a new study, an international research team reveal how fluoride nanoparticles, materials used in in vivo imaging, affect the assembly and structure of the amyloid β protein. Their results present a step towards better treatment and prevention of neurologic disorders like Alzheimer's disease.
Self-assembly, or the association of individual units of a material into ordered structures or patterns, is a phenomenon of great research interest for materials scientists. One prominent example of self-assembly comes from the self-assembly of proteins in biological systems. The function and activity of proteins are governed by their assembly state. Additionally, the protein's "secondary structure," characterized by its folding into structures, such as a β-sheet, also plays a role. In fact, abnormalities in the protein secondary structures or their assembly can lead to various neurodegenerative diseases, including Alzheimer's disease.
Nanoparticles (NPs) offer a promising route for the treatment and prevention of such diseases by allowing a controlled and targeted drug delivery. Additionally, inorganic NPs, such as fluoride NPs, are used in brain imaging applications. Compared to organic NPs, inorganic NPs are considered a better candidate for developing high functional materials. But, there is much concern regarding their bio-toxicity. While their interactions with bioproteins have been studied, the mechanism underlying these interactions are not well understood.
An international team of scientists from Tokyo University of Science (TUS) in Japan and Nazarbayev University in Kazakhstan has now addressed this issue. In their study, which was made available online on June 2, 2022, and was published in Volume 5, Issue 6 the journal ACS Applied Bio Materials on June 20, 2022, the team investigated a section of the amyloid β peptide (a protein found in the plaques forming in the brains of patients with Alzheimer's disease) in solution with fluoride ceramic (CeF3) NPs. The study was led by Junior Associate Professor Masakazu Umezawa and included contributions from Mr. Naoya Sakaguchi from TUS and Assistant Professors Mehdi Amouei Torkmahalleh and Dhawal Shah from Nazarbayev University.
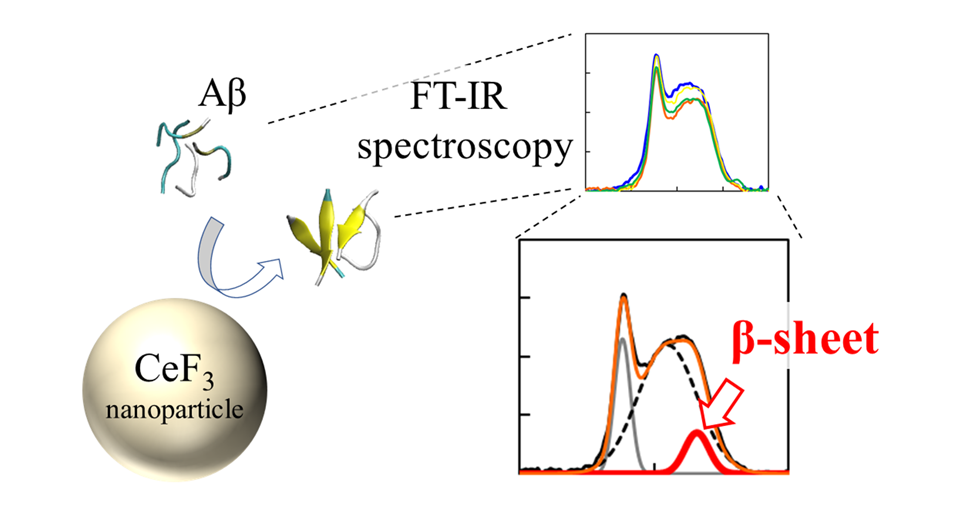
The team used a technique called "Fourier transform infrared spectroscopy" (FTIR) to directly monitor the effect of the NP surface on the peptide bonds. "We found that, near the nanoparticle surface, peptides are more likely to form β-sheets. This comes as an effect of hydrophobicity. The parts of the peptide that repelled by the water solution stick to the nanoparticles, and form aggregates more easily," explains Dr. Umezawa.
In addition, the team investigated the effect of other surrounding ions in the solution. "What we found was very surprising. Even without the nanoparticles, the environment affected the rate of secondary structure formation," says Dr. Umezawa, "This effect, resulting from a combination of electrostatic interaction and hydrogen bonding, was exaggerated upon adding nanoparticles. With a careful choice of ions and nanoparticles, the β-sheet formation can be either suppressed or promoted. This implies that the process can be controlled and engineered to eradicate adverse effects."
The experimental results were complemented with molecular dynamics simulations performed by the Nazarbayev University team. This, in turn, helped design and guide the experiments as well as provide insights into the results.
With this deeper understanding of the interaction between proteins and NPs, the study paves the way for controlled protein folding processes. With such control, any protein deformations could be eliminated, and positive interactions and structural changes could be promoted. This could lead to a better prevention and treatment protocol for Alzheimer's disease and, eventually, to a better quality of life for aged adults.
|
Figure title: Infrared (FT-IR) spectra and β-sheet ratio of Aβ peptides interacted with CeF3 NPs.
Figure Caption: The effect of CeF3 nanoparticles on amyloid beta protein structure is measured directly with FT-IR spectroscopy. The formation of secondary structure shows up as a feature in the IR spectrum.
|
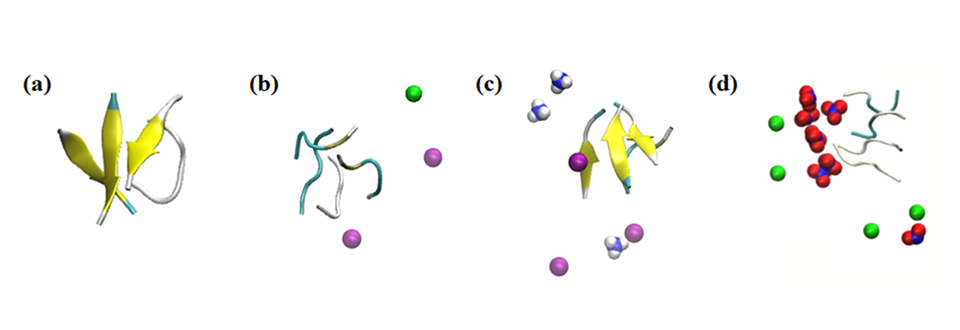
Figure title: Simulation snapshots of the peptide aggregate interactions with various ions.
Figure caption: Simulation results of the effect of ions within 0.1 nm on the peptides in the systems under study: (a) no salt, (b) 0.15 M NaCl, (c) 0.15 M NH4Cl, and (d) 0.15 M NaNO3. Coloring: beta sheet = yellow; Na+ = green; NH4+ = blue and white; Cl- = purple; and NO3− = blue and red.
Image credit: Masakazu Umezawa from Tokyo University of Science
Usage restrictions: Can be reused only with proper attribution
License type: CC BY 4.0
Image link: https://doi.org/10.1021/acsabm.2c00239 
Reference
| Title of original paper | : | Changes in the Secondary Structure and Assembly of Proteins on Fluoride Ceramic (CeF3) Nanoparticle Surfaces |
| Journal | : | ACS Applied Bio Materials |
| DOI | : | 10.1021/acsabm.2c00239  |
About The Tokyo University of Science
Tokyo University of Science (TUS) is a well-known and respected university, and the largest science-specialized private research university in Japan, with four campuses in central Tokyo and its suburbs and in Hokkaido. Established in 1881, the university has continually contributed to Japan's development in science through inculcating the love for science in researchers, technicians, and educators.
With a mission of "Creating science and technology for the harmonious development of nature, human beings, and society", TUS has undertaken a wide range of research from basic to applied science. TUS has embraced a multidisciplinary approach to research and undertaken intensive study in some of today's most vital fields. TUS is a meritocracy where the best in science is recognized and nurtured. It is the only private university in Japan that has produced a Nobel Prize winner and the only private university in Asia to produce Nobel Prize winners within the natural sciences field.



About Junior Associate Professor Masakazu Umezawa from Tokyo University of Science
Dr. Masakazu Umezawa is currently a Junior Associate Professor at Tokyo University of Science (TUS), Japan. He completed his PhD in Pharmaceutics and Drug Design in 2010 from TUS and held positions as a Postdoc and Project Research Associate there before becoming Junior Associate Professor in April 2021. His research interests include investigating nano-bio interactions, especially the structural change and assembly of proteins and lipids on the surfaces of inorganic and organic nanoparticles in physiological fluids. He aims to promote pharmaceutically effective and safer use of well-designed nanoparticles. Dr. Umezawa has published 100 peer-reviewed articles with over 1900 citations.
https://www.tus.ac.jp/en/fac/p/index.php?58D3 
https://sites.google.com/view/umelabsince2021/umelab-home 
Funding information
This study was funded by Tokyo University of Science: Faculty of Advanced Engineering Young Scientist Collaborative Research grant (2021) and Nazarbayev University: collaborative research project (11022021CRP1503).

