2022.06.13 Monday
Controlled Fabrication of Multimetallic Building Blocks for Hybrid Nanomaterials
The new method can be used to construct copolymers comprising different metal species, which have potential uses in catalysis and drug discovery
Polymers with different metal complexes in their side chains are thought to be promising high-performance materials with a wide variety of applications. However, conventional fabrication methods are not suitable for constructing such polymers because controlling their resulting metal composition is complicated. Recently, scientists from Japan have developed a method to overcome this limitation and successfully produce multimetallic copolymers, which can be used as building blocks to create future hybrid materials.
|
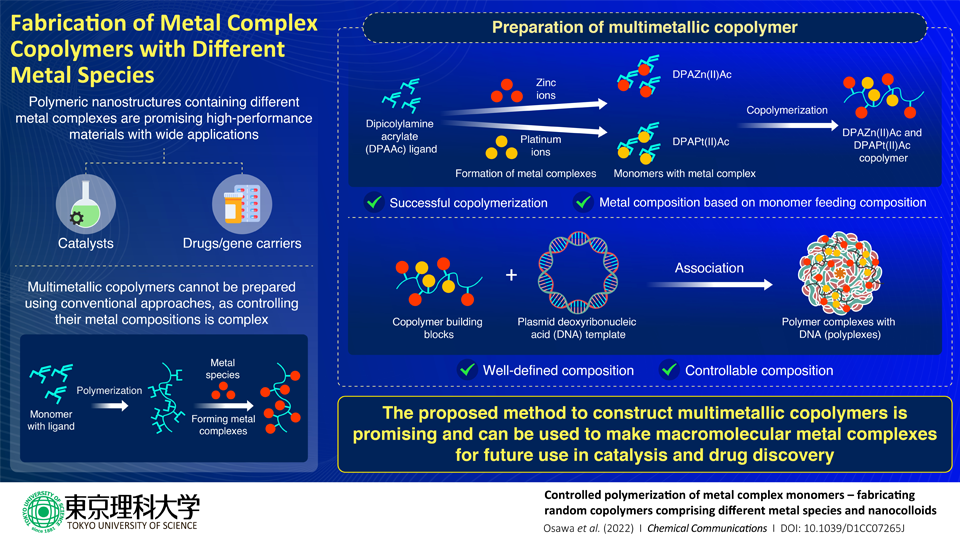
From plastics to clothes to DNA, polymers are everywhere. Polymers are highly versatile materials that are made of long chains of repeating units called monomers. Polymers containing metal complexes on their side chains have enormous potential as hybrid materials in a variety of fields. This potential only increases with the inclusion of multiple metal species into the polymers. But conventional methods of fabricating polymers with metal complexes are not appropriate for the construction of multimetallic polymers, because controlling the composition of metal species in the resulting polymer is complex.
Recently, a research team, led by Assistant Professor Shigehito Osawa and Professor Hidenori Otsuka from Tokyo University of Science, has proposed a new method of polymerization that can overcome this limitation. Dr. Osawa explains, "The usual method of preparing such complexes is to design a polymer with ligands (molecular 'backbones' that join together other chemical species) and then add the metal species to form complexes on it. But each metal has a different binding affinity to the ligand, which makes it complicated to control the resulting structure. By considering polymerizable monomers with complexes of different metal species, we can effectively control the composition of the resulting copolymer." The study was made available online on April 1, 2022, and published in Volume 58, Issue 34 of Chemical Communications on April 30, 2022.
When the monomers that make up a polymer are polymers themselves, the polymer is called a copolymer. For their study, the scientists designed a dipicolylamine acrylate (DPAAc) monomer. DPA was chosen because it is an excellent metal ligand and has been used in various biochemical applications. They then polymerized DPAAc with zinc (Zn) and platinum (Pt) to form two polymer chains with metal complexes―DPAZn(II)Ac and DPAPt(II)Ac. They then copolymerized the two monomers. They found that they could not only successfully create a copolymer, but that they could also control its metal composition by varying the feeding composition of the monomers.
Then they applied this copolymer as a building block to fabricate nanoparticles using plasmid deoxyribonucleic acid (DNA) as a template. Plasmid DNA was chosen as a template because the two constituent monomers are known to bind to it. The formation of the resulting nanoparticle polymer complexes with DNA (polyplexes) was confirmed using high-resolution scanning tunneling electron microscopy and energy-dispersive X-ray spectroscopy.
This technique―now a patent-pending technology―can be extended to a novel method for fabricating intermetallic nanomaterials. "Intermetallic catalytic nanomaterials are known to have significant advantages over nanomaterials containing only a single metallic species," says Dr. Osawa.
The polyplexes formed in the study are DNA-binding molecules, which indicates that they could be used to develop anti-cancer drugs and gene carriers. The proposed fabrication method will also lead to advances in catalysis that move away from precious metals like platinum. "These multimetallic copolymers can serve as building blocks for future macromolecular metal complexes of many varieties," concludes Dr. Osawa.
The findings of this study are sure to have far reaching consequences in the field of polymer chemistry.
Reference
| Title of original paper | : | Controlled polymerization of metal complex monomers - fabricating random copolymers comprising different metal species and nano-colloids |
| Journal | : | Chemical Communications |
| DOI | : | 10.1039/D1CC07265J |
About The Tokyo University of Science
Tokyo University of Science (TUS) is a well-known and respected university, and the largest science-specialized private research university in Japan, with four campuses in central Tokyo and its suburbs and in Hokkaido. Established in 1881, the university has continually contributed to Japan's development in science through inculcating the love for science in researchers, technicians, and educators.
With a mission of "Creating science and technology for the harmonious development of nature, human beings, and society", TUS has undertaken a wide range of research from basic to applied science. TUS has embraced a multidisciplinary approach to research and undertaken intensive study in some of today's most vital fields. TUS is a meritocracy where the best in science is recognized and nurtured. It is the only private university in Japan that has produced a Nobel Prize winner and the only private university in Asia to produce Nobel Prize winners within the natural sciences field.
About Assistant Professor Shigehito Osawa from Tokyo University of Science
Shigehito Osawa obtained a PhD in Materials Engineering from the University of Tokyo, Japan, in 2016. He worked as a Research Scientist at the Kawasaki Institute of Industrial Promotion from 2016 to 2018. He joined Tokyo University of Science afterwards, where he now serves as Assistant Professor at the Department of Applied Chemistry. His research interests are in the fields of polymer materials and polymer chemistry. He has published 24 peer-reviewed papers and has patent-pending technology currently under review. He is currently a member of the Water Frontier Research Center (WaTUS).
About Professor Hidenori Otsuka from Tokyo University of Science
https://www.tus.ac.jp/en/fac/p/index.php?3f8f 
https://www.rs.kagu.tus.ac.jp/otlabo/index.html 
Funding information
This work was financially supported by Grants-in-Aids for Early Carrier Scientists (JSPS KAKENHI Grant Number 20K15346 to Shigehito Osawa) from the Japanese Society of the Promotion of Science (JSPS).

