2021.11.25 Thursday
Immune Cell Receptor and Ligand Regulation: A Therapeutic Avenue for Inflammatory Diseases
Tohoku Medical and Pharmaceutical University
Tokyo University of Science
Researchers identify a novel regulatory axis targeting dendritic cell activity and subsequent inflammatory responses in immune disorders
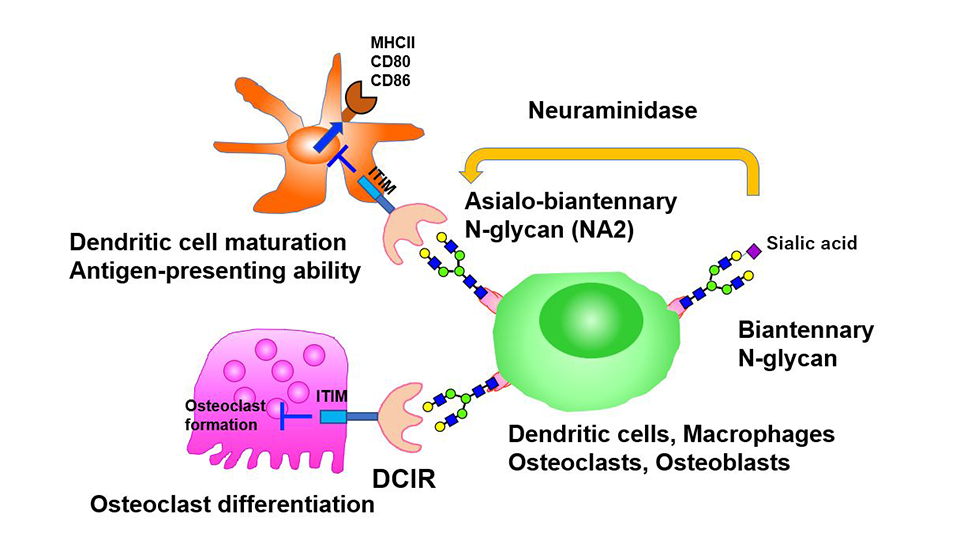
While dendritic cell immunoreceptor (DCIR) is known to mediate inflammation and bone metabolism, ligands that bind DCIR and the mechanisms underlying DCIR activity remain poorly understood. Researchers from Japan have now identified "asialo-biantennary N-glycan"—a glycoprotein present on the surface of bone cells and dendritic cells, as a functional ligand of DCIR. Their findings could help in understanding DCIR's role in the pathogenesis of autoimmune diseases and bone disorders and develop novel targeted therapies.
Immune cells play a key role in mediating inflammatory responses. Dysregulation in signaling mechanisms that operate across immune cells can trigger chronic inflammatory diseases like rheumatoid arthritis that cause pain and swelling in joints. One such immune cell known to be involved in autoimmune diseases is the dendritic cell. The activity of dendritic cells is regulated by the dendritic cell immunoreceptor (DCIR) present on their surface, which comprises a carbohydrate recognition domain that can bind to sugar moieties present on other proteins or cell surfaces, in a calcium dependent manner. The activity of osteoclast, which is involved in bone degradation, is also regulated by DCIR. However, little is known about the interacting partners of DCIR that help mediate inflammatory responses.
A team of researchers from Japan led by Professor Yoichiro Iwakura of the Department of Experimental Animal Science at the Research Institute for Biomedical Sciences, Tokyo University of Science, have now delved deeper into understanding the mechanisms underlying DCIR activity. In their previous work, the researchers reported that mice deficient in DCIR spontaneously develop arthritis and metabolic bone disorders. Building on this finding, in a recent study published in the Journal of Experimental Medicine, they sought to elucidate DCIR binding partners and immune signaling mechanisms involved in inflammatory diseases. "In this study, we have identified a novel functional ligand of DCIR, likely involved in the pathogenesis of arthritis and other autoimmune diseases like multiple sclerosis. We are hopeful that our work can advance the research of immunology and glycobiology in inflammatory diseases.", explains Prof. Iwakura.
The researchers began by identifying potential ligands (molecules that bind to cell receptors) of DCIR on immune and bone cells, and found that DCIR binds to glycoproteins present on the surface of macrophages and osteoclasts, the latter differentiating from bone marrow derived macrophages (BMMs), and involved in bone degeneration and remodeling. On further characterization of the glycoprotein, they noted that this interaction was specific to "asialo-biantennary N-glycan (NA2)," a complex carbohydrate moiety comprising various sugar molecules.
Having identified the DCIR ligand, the team next sought to understand the effect of DCIR on osteoclast differentiation and "osteoclastogenesis," a process contributing to bone loss. Interestingly, cells deficient in DCIR showed a significant increase in the expression of osteoclastogenesis associated genes. As the researchers speculated, expression of DCIR significantly suppressed the differentiation of osteoclasts, thus suggesting DCIR as an independent inhibitor of osteoclastogenesis. Further confirming this finding, a mutant version of DCIR, incapable of recognizing carbohydrate residues, was shown to not exhibit this inhibitory effect.
The role of DCIR in osteoclast differentiation, and its interacting ligand, NA2, now decoded, the team next examined the effect of NA2 on osteoclastogenesis. Consistent with their previous finding, NA2 treatment suppressed the differentiation of osteoclasts from wild-type BMMs but not from cells deficient in DCIR, underscoring the role of NA2 as a specific functional ligand of DCIR that suppresses osteoclastogenesis.
Taking a step further, the researchers treated mouse autoimmune disease models with neuraminidase, an enzyme that removes terminal "sialic acid" residues from N-glycan, thereby enhancing the exposure of NA2. Much to their delight, neuraminidase treatment further suppressed autoimmune diseases like autoimmune arthritis or experimental autoimmune encephalomyelitis, yet again, in a DCIR dependent manner! Furthermore, neuraminidase treatment ameliorated inflammation and associated bone loss in a mouse model of arthritis, thereby confirming their findings in vitro and in vivo. The inhibitory effect of DCIR-NA2 interaction on autoimmune diseases was found to be mediated through suppression of the antigen presenting ability of dendritic cells and subsequent decrease in the activation of other immune cells that contribute to inflammation.
All these findings, together, highlight a novel regulatory mechanism of DCIR signaling involved in the suppression of autoimmunity and excess bone loss. Commenting on the clinical applications of their work, Prof. Iwakura observes, "Our findings are expected to contribute to the understanding of the pathogenesis of human autoimmune diseases such as rheumatoid arthritis and to the development of novel therapies for the treatment of immune and bone metabolic diseases."
This study indeed takes us a step closer to resolving the complex immune mechanisms of autoimmune diseases, thus paving the way towards effective and targeted treatments.
|
Schematic representation of the activity of the dendritic cell immunoreceptor during inflammation
Neuraminidase suppresses autoimmune arthritis and encephalomyelitis by inhibiting dendritic cell and osteoclast activity through removal of sialic acid, thereby exposing asialo-biantennary N-glycan (NA2) and generating inhibitory signals through dendritic cell immunoreceptor (DCIR).
Image courtesy: Yoichiro Iwakura from Tokyo University of Science
Reference
| Authors | : | Tomonori Kaifu1,2,5, Rikio Yabe1,5, Takumi Maruhashi1,3,5, Soo-Hyun Chung1,2,5, Hiroaki Tateno4, Noriyuki Fujikado1,2, Jun Hirabayashi4 and Yoichiro Iwakura1,2,6 |
| Title of original paper | : | DCIR and its ligand asialo-biantennary N-glycan regulate DC function and osteoclastogenesis |
| Journal | : | Journal of Experimental Medicine |
| DOI | : | 10.1084/jem.20210435 |
| Affiliations | : |
1Center for Animal Disease Models, Research Institution for Biological Sciences, Tokyo University of Science 2Core Research for Evolutional Science and Technology (CREST), Japan Science and Technology Agency 3Japan Society for the Promotion of Science (JSPS) 4Glycan Lectin Engineering Team, Research Center for Stem Cell Engineering, National Institute of Advanced Industrial Science and Technology (AIST) 5These authors are equal contributors to this work. 6Corresponding author Current address: Tomonori Kaifu: Division of Immunology, Faculty of Medicine, Tohoku Medical and Pharmaceutical University Rikio Yabe: Division of Molecular Immunology, Medical Mycology Research Center, Chiba University Takumi Maruhashi: Laboratory of Molecular Immunology, Institute for Quantitative Biosciences, The University of Tokyo Noriyuki Fujikado: Lilly Research Laboratories, Lilly Biotechnology Center, Eli Lilly and Company Jun Hirabayashi: Tokai National Higher Education and Research System, Nagoya University |
Further Information
Professor Yoichiro Iwakura
Center for Animal Disease Models, Research Institution for Biological Sciences
Tokyo University of Science
Email: iwakura【@】rs.tus.ac.jp
Lecturer Tomonori Kaifu
Division of Immunology, Faculty of Medicine
Tohoku Medical and Pharmaceutical University
Email: kaifu【@】tohoku-mpu.ac.jp
Media contact
Tsutomu Shimizu
Public Relations Divisions
Tokyo University of Science
Email: mediaoffice【@】admin.tus.ac.jp
Website: https://www.tus.ac.jp/en/mediarelations/ 
Yukiyo Sekine
Public Relations Office
Tohoku Medical and Pharmaceutical University
E-mail: koho【@】tohoku-mpu.ac.jp
Website: https://www.tohoku-mpu.ac.jp/english/ 
About The Tokyo University of Science
Tokyo University of Science (TUS) is a well-known and respected university, and the largest science-specialized private research university in Japan, with four campuses in central Tokyo and its suburbs and in Hokkaido. Established in 1881, the university has continually contributed to Japan's development in science through inculcating the love for science in researchers, technicians, and educators.
With a mission of "Creating science and technology for the harmonious development of nature, human beings, and society", TUS has undertaken a wide range of research from basic to applied science. TUS has embraced a multidisciplinary approach to research and undertaken intensive study in some of today's most vital fields. TUS is a meritocracy where the best in science is recognized and nurtured. It is the only private university in Japan that has produced a Nobel Prize winner and the only private university in Asia to produce Nobel Prize winners within the natural sciences field.
About Professor Yoichiro Iwakura from Tokyo University of Science
Professor Yoichiro Iwakura has been the director of the Center for Animal Disease Models at the Tokyo University of Science since 2013, and has published more than 670 papers since his graduation from Kyoto University in 1970. He started researching interferon proteins at the university's Institute for Virus Research before moving to the Sloan-Kettering Cancer Institute in the United States to analyze the developmental mechanism of early mouse embryos. In 1985, he moved to the University of Tokyo where he generated more than 100 lines of gene-modified mice as the director of the Center for Experimental Medicine to analyze the pathogenesis of infectious and autoimmune diseases. He retired from the University of Tokyo and became an Emeritus Professor in 2012. Then, he moved to the Tokyo University of Science. He has been a visiting professor for many universities including Dalian Medical University in China and Chiba University in Japan. His work involves the development and analysis of animal disease models and research on autoimmune diseases and infectious diseases. He won the Hideyo Noguchi Memorial Award for Medical Science in 2015 and was selected as a highly cited researcher (Thomson Reuters and Clarivate Analytics) for 6 years from 2014.
https://www.tus.ac.jp/en/fac/p/index.php?657F 
https://www.rs.tus.ac.jp/iwakuralab/ 
Funding information
This study was supported in part by grants for the CREST from Japan Science and Technology Agency (JST) (Grant Number, 105100000222), Grant-in-Aid for Scientific Research (S) (24220011) and Grant-in-Aid for Scientific Research on Innovative Areas (20H04954)from Japan Society for the Promotion of Science (JSPS), a grant from Japan Agency for Medical Research and Development (AMED) (16809407), and a Grant-in-Aid for Scientific Research (C) (23500489) from JSPS.

