2020.02.05 Wednesday
How manipulating ligand interactions in metal clusters can spur advances in nanotechnology
Researchers uncover how interactions among ligands dictate the final structure of metal clusters, which have various applications in modern electronic devices
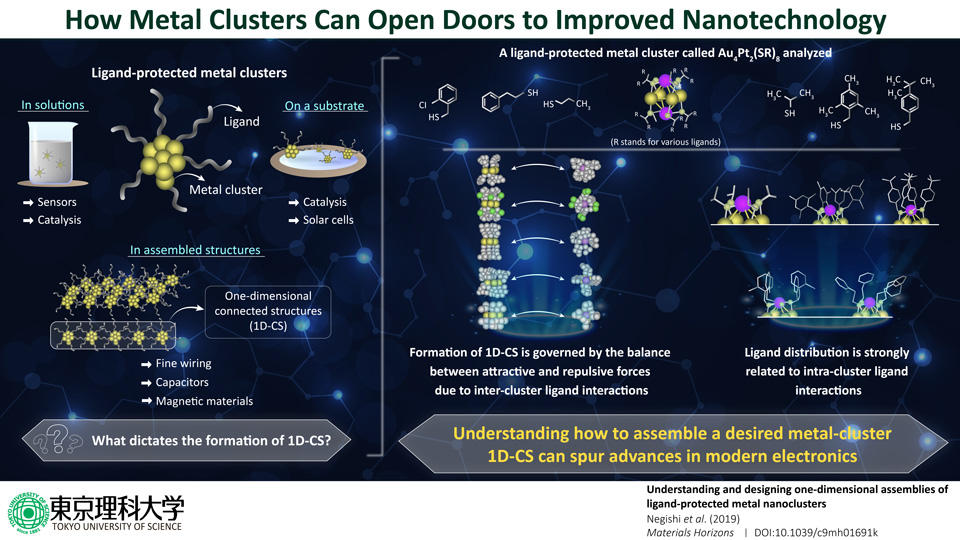 |
Ligand-protected metal clusters in assembled structures show peculiar properties, which are different from those of corresponding bulk metals. These properties have promising applications in a wide variety of fields, especially nanotechnology and materials science. In a recent study, researchers from Tokyo University of Science reveal the main factors that influence how assembled structures are formed from metal clusters.
When metal atoms form small clusters of a particular size, they show interesting and potentially useful electromagnetic characteristics, which are different from those of the actual bulk metal. To fully explore the potential of these properties, it is necessary to find ways to assemble precise macroscopic structures out of these clusters. But, how do these clusters bind together, and what exactly dictates their properties? These questions have remained unanswered, until now. In a new study published in Materials Horizons, researchers from the Tokyo University of Science, led by Prof Yuichi Negishi, set out to find these answers. Prof Negishi explains the motivation behind this study, "Previous studies have found that gold clusters can form one-dimensional connected structures (1D-CS) that are linked via a single gold atom in each cluster. While assembling ligand-protected metal clusters is an interesting approach to realize new physical properties and functions, the factors required for the formation of 1D-CS are currently poorly understood." This exciting new research paper has been selected to be on the cover of the next issue of the journal.
To begin with, the researchers wanted to see how intra-cluster ligand interactions dictate the formation of metal clusters. For this, they focused on a particular type of ligand-protected metal cluster called "thiolate (SR)-protected gold-platinum alloy cluster ([Au4Pt2(SR)8]0)", as it had various types of ligand distributions. Through techniques like single crystal X-ray structural analysis, the researchers found that these metal clusters attach to one another via gold atom bonds, and these bonds form 1D-CS depending on the attractive and repulsive forces caused by inter-cluster ligand interactions. They also found that these interactions are affected by how ligands are distributed on the clusters and the angles that they form. More specifically, when ligands were uniformly spread around the metal cluster (which indicates repulsive forces between ligands), the repulsive forces between different metal clusters were also higher, thereby preventing the formation of 1D-CS. Prof Negishi explains, "We found that the ligand distribution in [Au4Pt2(SR)8]0 changes depending on the ligand structure and that differences in the distributions of the ligands influence the inter-cluster ligand interactions. Thus, we need to design intra-cluster ligand interactions to produce 1D-CS with desired connecting structures." In fact, through further analyses, the scientists even found that the formation of 1D-CS had an effect on the overall electronic structure of the metal clusters, even affecting their conductivity.
These findings serve as guidelines for those trying to create 1D-CS to leverage the potential of metal cluster assemblies. One notable application of metal clusters, states Prof Negishi, is the fabrication of finer wiring. He says, "It is challenging to draw finer wiring using conventional top-down technology; fortunately, progress in the field of metal nanoclusters will enable the development of bottom-up technology for drawing finer wiring."
This study paves the way for significant improvements in sophisticated electronics systems and devices. Not just this, these findings will act as a beacon for scientists working in the fields of nanotechnology and nanoengineering!
Reference
| Title of original paper | : | Understanding and designing one-dimensional assemblies of ligand-protected metal nanoclusters |
| Journal | : | Materials Horizons |
| DOI | : | 10.1039/c9mh01691k |
About The Tokyo University of Science

Tokyo University of Science (TUS) is a well-known and respected university, and the largest science-specialized private research university in Japan, with four campuses in central Tokyo and its suburbs and in Hokkaido. Established in 1881, the university has continually contributed to Japan's development in science through inculcating the love for science in researchers, technicians, and educators.
With a mission of "Creating science and technology for the harmonious development of nature, human beings, and society", TUS has undertaken a wide range of research from basic to applied science. TUS has embraced a multidisciplinary approach to research and undertaken intensive study in some of today's most vital fields. TUS is a meritocracy where the best in science is recognized and nurtured. It is the only private university in Japan that has produced a Nobel Prize winner and the only private university in Asia to produce Nobel Prize winners within the natural sciences field.
About Professor Jun Taniguchi from Tokyo University of Science
Professor Yuichi Negishi has dedicated his career to physical and nanomaterials chemistry. He graduated from Keio University, where he also acquired his PhD in 2001. After working as a research associate in both Keio University and the Institute for Molecular Science, he joined the Tokyo University of Science as a junior associate professor in 2008, becoming a professor in 2017. As of 2018, he is also Dean of Graduate School and Head of Department. Many of his research publications in nanotechnology and materials science are highly regarded by scientific journals and selected for cover/back covers or featured articles.
PROFILE
Negishi Yuichi Professor
Tokyo University of Science, Faculty of Science Division I, Department of Applied Chemistry
https://www.tus.ac.jp/en/fac/p/index.php?5825
Funding information
This work was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI (grant nos. JP16H04099 and 16K21402) and Grants-in-Aid for Scientific Research on Innovative Areas ''Coordination Asymmetry'' (grant no. 17H05385) and ''Innovations for Light-Energy Conversion'' (grant no. 18H05178). Funding from Asahi Glass Foundation is also acknowledged.

