Solid oxide fuel cells (SOFC) are power generation units that use hydrogen as fuel and produce electricity through chemical reaction with oxygen. Given that the heat generated at the same time as the electricity can also be put to effective use, as well as the fact that they have other advantages such as no CO2 emissions or battery fluid waste, SOFC have been attracting attention as an energy-efficient source of clean energy.
In recent years, there has been a great deal of research conducted into the electrolytic/electrode materials used in SOFC. One issue for SOFC is their limited application, due to the fact that they operate at high temperatures between about 700℃ and 1000℃; thus, there is need for materials that will allow them to operate at lower temperatures.
The research group of Associate Professor Tohru Higuchi in the Faculty of Science Division I Department of Applied Physics is working on developing SOFC electrolytic/electrode materials. SOFC can be roughly divided into two types: those that utilize “oxygen ion conductivity” and those that utilize “proton (hydrogen ion) conductivity.” The latter is the type that Associate Professor Higuchi’s group is focused on.
Compared with oxygen ion-conductive SOFC, proton-conductive SOFC have higher power density and fuel utilization when generating electricity. SOFC have two terminals, an “air terminal” (+) and a “fuel terminal” (-); with proton-conductive SOFC, high electromotive force can be maintained by lowering the rate of oxygen utilization at the air terminal.
The Higuchi Research Group has spent many years researching electrolytic/electrode materials that are able to operate at moderately low temperatures (300-600℃). From this research was developed, in 2020, an extremely thin film SDC electrolyte material.
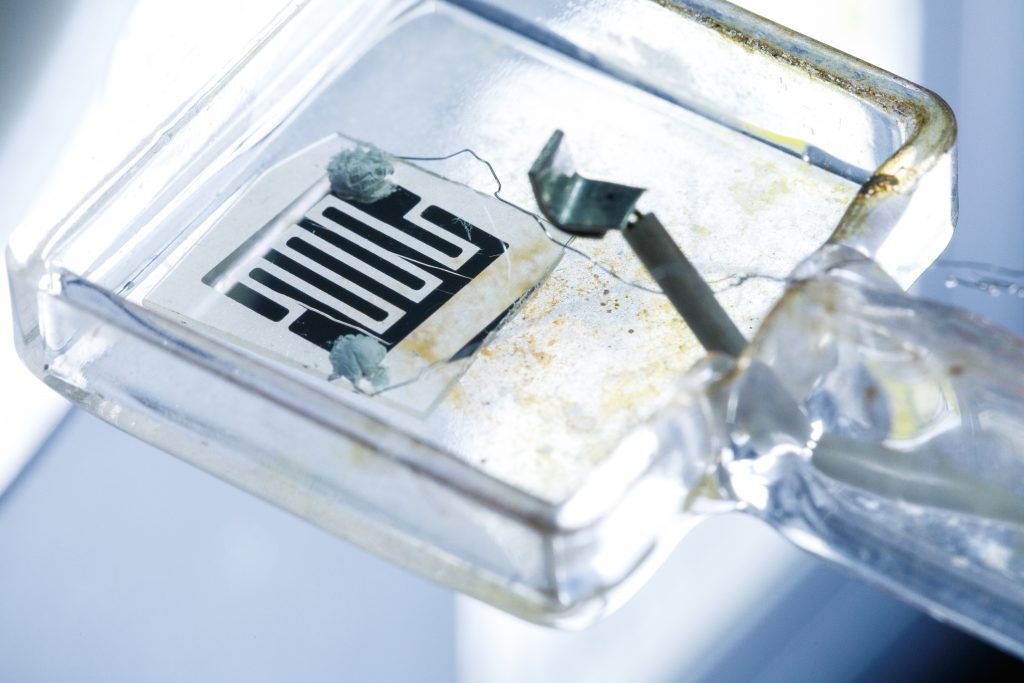
For SOFC, it has always been difficult with conventional fuel to achieve sufficient proton conduction at lower temperatures. The research group worked to improve upon this by focusing on SDC thin film surface defects and crystal lattice modification. This enabled them to successfully achieve proton conductivity within a sub-100℃ temperature range.
Using these thin film SDC will make it possible to operate SOFC at normal temperatures while making the cells extremely small and thin. Thanks to the expansion in SOFC-related applications and reduction in SOFC-related costs provided by this material, it is having quite an impact globally, with research result already being announced in twelve different countries.
There is anticipation that SOFC will become a power generation system capable of replacing nuclear and thermal power generation. And the results of the Higuchi Research Group are furthering global progress towards the establishment of a safe and inexpensive energy supply.
■ Main research content