東京理科大学研究推進機構総合研究院(Research Institute for Science & Technology)は、英語の頭文字をとって
「RIST」(リスト)と呼ばれています。異なる学問分野の教員が連携し、学際型・分野横断型の研究を推進する組織です。
4 研究センター、1 共同利用・共同研究拠点、2 研究拠点、19 研究部門、2 共創プロジェクトが活動しています。(2023年8月現在)
-

研究センター Research Centers
国、地方公共団体、産業界等から活動に必要な研究費を獲得し、本学における研究戦略上重要な取り組みを行う研究組織
-

共同利用・
共同研究拠点 Joint Usage / Research Center文部科学省より共同利用・共同研究拠点として認定され、学外の研究者と行う先端的共同研究拠点
-

研究拠点 Research Hubs
本学の教員の叡智を駆使し、分野や組織を横断する研究の中核となる組織
-
研究部門 Research Divisions
本学及び学外の選抜された研究者によって構成され、シナジー効果を発揮し、学際的・分野横断的な「理科大ならでは」(Only at TUS)の研究活動をする中核的研究組織
-

共創プロジェクト The Open Innovation Projects
本学と企業による社会貢献を目指した新たな価値の共創を推進する研究組織
RISTの領域
「理科大ならでは」(Only at TUS)の研究成果実現をめざし、共通の研究テーマをもつRISTのグループです。
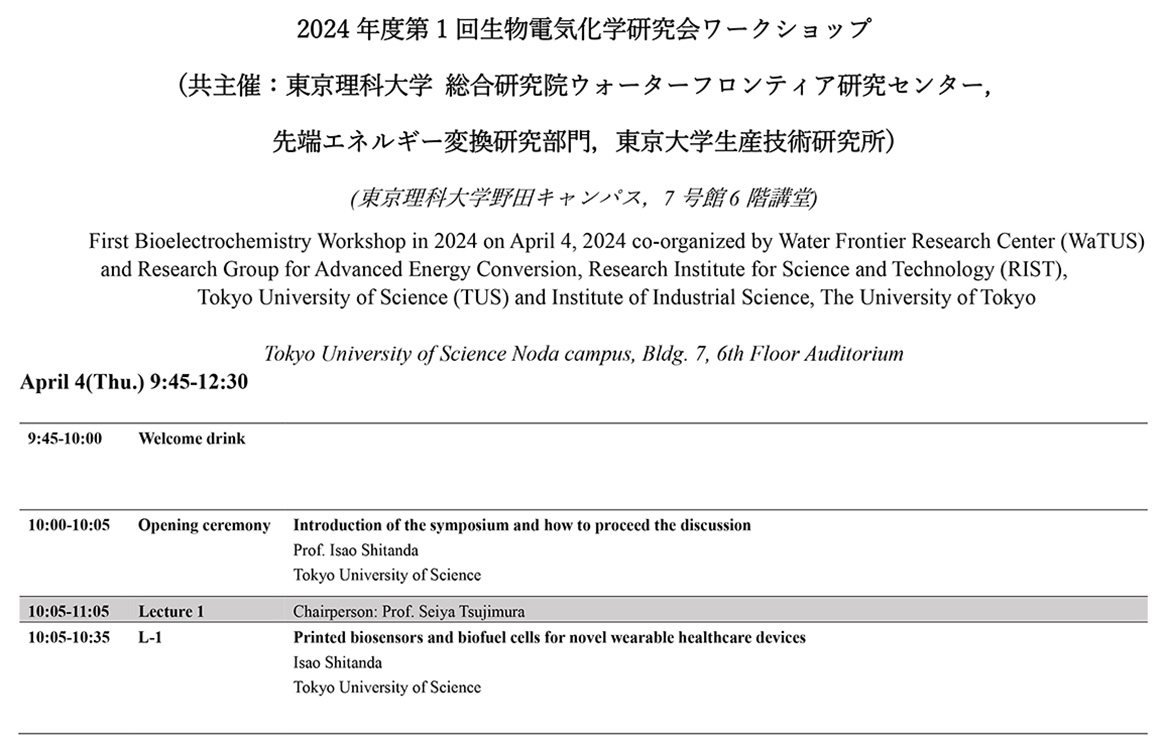
環境・情報・社会 / 基礎・計測 / 物質・材料 / 構造材料・機械・流体・建築 / 創薬・バイオの5つからなり、
どのセクションもいずれかに属しています。

各センター・拠点・部門紹介 Introduction
研究センター、共同利用・共同研究拠点、研究拠点、研究部門、共創プロジェクトの研究内容やメンバーなどの詳細を紹介しています。

メンバー Members
RIST所属の本務教員を紹介します。
それぞれの活動報告をさまざまな形で発表・紹介をしています。